
Organic Chemistry
4th Edition
ISBN: 9780073402772
Author: Janice G. Smith
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 5.58P
Explain each statement by referring to compounds A-E.
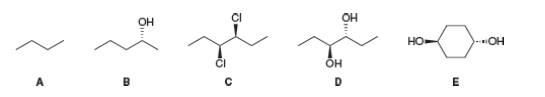
a. A has a mirror image bur no enantiomer.
b. B has an enantiomer and no diastereomer.
c. C has both an enantiomer and a diastereomer.
d. D has a diastereomer but no enantiomer.
e. E has a diastereomer but no enantiomer.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Alcohols can be synthesized using an acid-catalyzed hydration of an alkene. An alkene is combined with aqueous acid (e.. sulfuric acid in water). The
reaction mechanism typically involves a carbocation intermediate.
>
3rd attempt
3343
10
8
Draw arrows to show the reaction between the alkene and hydronium ion.
that
2nd attempt
Feedback
1st attempt
تعمال
Ju See Periodic Table See Hint
F
D
Ju See Periodic Table See Hint
Draw the simplified curved arrow mechanism for the reaction of acetone and CHgLi to give the major product.
4th attempt
Π
Draw the simplified curved arrow mechanism
T
3rd attempt
Feedback
Ju
See Periodic Table See Hint
H
-H
H
-I
H
F
See Periodic Table See Hint
Select the correct reagent to accomplish the first step of this reaction. Then draw a mechanism on the Grignard reagent using curved arrow notation
to show how it is converted to the final product.
4th attempt
Part 1 (0.5 point)
Select the correct reagent to accomplish the first step of this reaction.
Choose one:
OA Mg in ethanol (EtOH)
OB. 2 Li in THF
O C. Li in THF
D. Mg in THF
O E Mg in H2O
Part 2 (0.5 point)
Br
Part 1
Bri
Mg
CH
B
CH,
1 Draw intermediate here, but no arrows.
©
TE
See Periodic Table See Hint
See Hint
ין
H
Chapter 5 Solutions
Organic Chemistry
Ch. 5 - Prob. 5.1PCh. 5 - Prob. 5.2PCh. 5 - Draw the mirror image of each compound. Label each...Ch. 5 - Prob. 5.4PCh. 5 - Prob. 5.5PCh. 5 - Prob. 5.6PCh. 5 - The facts in section 5.4A can be used to locate...Ch. 5 - Prob. 5.8PCh. 5 - Prob. 5.9PCh. 5 - Prob. 5.10P
Ch. 5 - Prob. 5.11PCh. 5 - Prob. 5.12PCh. 5 - Label each compound as R or S.Ch. 5 - Prob. 5.14PCh. 5 - Prob. 5.15PCh. 5 - Prob. 5.16PCh. 5 - Prob. 5.17PCh. 5 - Prob. 5.18PCh. 5 - Prob. 5.19PCh. 5 - Prob. 5.20PCh. 5 - Prob. 5.21PCh. 5 - Prob. 5.22PCh. 5 - Prob. 5.23PCh. 5 - Which of the following cyclic molecules are meso...Ch. 5 - Prob. 5.25PCh. 5 - Prob. 5.26PCh. 5 - The amino acid (S)-alanine has the physical...Ch. 5 - Prob. 5.28PCh. 5 - Prob. 5.29PCh. 5 - Prob. 5.30PCh. 5 - Prob. 5.31PCh. 5 - Prob. 5.32PCh. 5 - Prob. 5.33PCh. 5 - Prob. 5.34PCh. 5 - Prob. 5.35PCh. 5 - Prob. 5.36PCh. 5 - Prob. 5.37PCh. 5 - Determine if each compound is identical to or an...Ch. 5 - Prob. 5.39PCh. 5 - Locate the stereogenic centers in each compound. A...Ch. 5 - Prob. 5.52PCh. 5 - Prob. 5.41PCh. 5 - Prob. 5.42PCh. 5 - Prob. 5.43PCh. 5 - Prob. 5.44PCh. 5 - Prob. 5.45PCh. 5 - Label each stereogenic center as R or S. a. c. e....Ch. 5 - Prob. 5.47PCh. 5 - Prob. 5.48PCh. 5 - Prob. 5.49PCh. 5 - Prob. 5.50PCh. 5 - Prob. 5.51PCh. 5 - Prob. 5.53PCh. 5 - Prob. 5.54PCh. 5 - Prob. 5.55PCh. 5 - Draw all possible stereoisomers for each...Ch. 5 - Prob. 5.57PCh. 5 - 5.59 Explain each statement by referring to...Ch. 5 - Prob. 5.59PCh. 5 - Prob. 5.60PCh. 5 - Prob. 5.61PCh. 5 - Prob. 5.62PCh. 5 - Prob. 5.63PCh. 5 - Prob. 5.64PCh. 5 - Prob. 5.65PCh. 5 -
5.67 Artemisinin and mefloquine are widely used...Ch. 5 - 5.68 Saquinavir (trade name Invirase) is a...Ch. 5 - Prob. 5.68PCh. 5 - Prob. 5.69PCh. 5 - Prob. 5.70PCh. 5 - Prob. 5.71PCh. 5 - Problem 5.73 An acid-base reaction of with a...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Sea turtles have disappeared from many regions, and one way of trying to save them is to reintroduce them to ar...
MARINE BIOLOGY
Some people compare DNA to a blueprint stored in the office of a construction company. Explain how this analogy...
Biology: Concepts and Investigations
Gregor Mendel never saw a gene, yet he concluded that some inherited factors were responsible for the patterns ...
Campbell Essential Biology (7th Edition)
What process causes the Mediterranean intermediate Water MIW to become more dense than water in the adjacent At...
Applications and Investigations in Earth Science (9th Edition)
Why do scientists think that all forms of life on earth have a common origin?
Genetics: From Genes to Genomes
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Select the product for the following reaction. HO HO PCC OH ○ OH O HO ○ HO HO HOarrow_forward5:45 Х Select the final product for the following reaction sequence. O O 1. Mg. ether 2.D.Oarrow_forwardBased on the chart Two similarities between the molecule with alpha glycosidic linkages. Two similarities between the molecules with beta glycosidtic linkages. Two differences between the alpha and beta glycosidic linkages.arrow_forward
- please help fill in the tablearrow_forwardAnswer F pleasearrow_forward4. Refer to the data below to answer the following questions: The octapeptide saralasin is a specific antagonist of angiotensin II. A derivative of saralasin is used therapeutically as an antihypertensive. Amino acid analysis of saralasin show the presence of the following amino acids: Ala, Arg, His, Pro, Sar, Tyr, Val, Val A.Sar is the abbreviation for sarcosine, N-methyl aminoethanoic acid. Draw the structure of sarcosine. B. N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Tyr-Val-His Sar-Arg-Val His-Pro-Ala Val-Tyr-Val Arg-Val-Tyr What is the structure of saralasin?arrow_forward
- What is the structure of the DNA backbone?arrow_forwardPLEASE PLEASE PLEASE use hand drawn structures when possarrow_forward. M 1- MATCH each of the following terms to a structure from the list below. There is only one correct structure for each term and structures may be used more than once. Place the letter of the structure in the blank to the left of the corresponding term. A. Sanger dideoxy method C. Watson-Crick B. GAUCGUAAA D. translation E. HOH2C OH OH G. transcription I. AUGGCUGAG 0 K. OPOH2C 0- OH N- H NH2 F. -OPOH2C 0- OH OH H. Maxam-Gilbert method J. replication N L. HOH2C a. b. C. d. e. f. g. B M. AGATCGCTC a pyrimidine nucleoside RNA base sequence with guanine at the 3' end. DNA base sequence with cytosine at the 3' end. a purine nucleoside DNA sequencing method for the human genome 2'-deoxyadenosine 5'-phosphate process by which mRNA directs protein synthesis OH NH2arrow_forward
- Please use hand drawn structures when neededarrow_forwardB. Classify the following amino acid. Atoms other than carbon and hydrogen are labeled. a. acidic b. basic C. neutral C. Consider the following image. Which level of protein structure is shown here? a. primary b. secondary c. tertiary d. quaternary D. Consider the following image. H RH H HR H R HR HR RH Which level of protein structure is shown in the box? a. primary b. secondary R c. tertiary d. quaternary コー Rarrow_forwardBriefly answer three from the followings: a. What are the four structures of the protein? b. Why is the side chain (R) attached to the alpha carbon in the amino acids is important for the function? c. What are the types of amino acids? And how is it depend on the (R) structure? d. Write a reaction to prepare an amino acid. prodarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License