
Concept explainers
Draw all possible stereoisomers for each cycloalkane. Label pairs of enantiomers and diastereomers. Label any meso compound.
a. 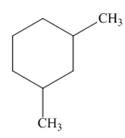 b.
b. 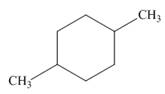 c.
c. 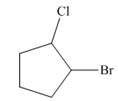
(a)
Interpretation: The possible stereoisomers for the given cycloalkane are to be drawn. The labeling of any enantiomers, diastereomers or meso compound is to be stated.
Concept introduction: A carbon atom that has four nonequivalent atoms or groups attached to it is known as chiral carbon atom. Chiral carbon centers are also called as asymmetric or stereogenic centers.
The stereocenters are carbon atoms on which the interchanging of two atoms or groups results in the formation of new stereoisomers. The stereocenters are also known as stereogenic centers.
Answer to Problem 5.56P
The possible stereoisomers for the given cycloalkane are three. Among the three stereoisomers, the labeling of two enantiomers of the given cycloalkane is
Explanation of Solution
The given cycloalkane is,
Figure 1
Due to the presence of cyclohexane ring attached with two methyl groups, the IUPAC name of this cycloalkane is
Where,
•
Substitute the number of chiral centers of
Thus, the total number of stereoisomers of
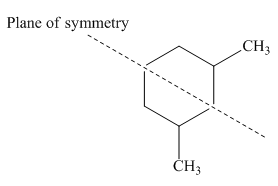
Figure 2
Thus, this compound forms only three stereoisomers instead of four. Among the three stereoisomers of
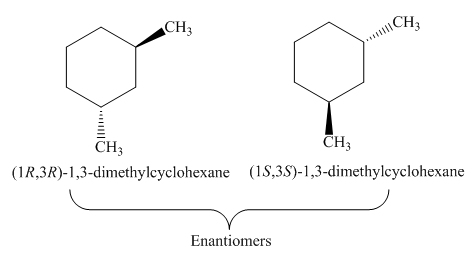
Figure 3
The remaining one stereoisomer of
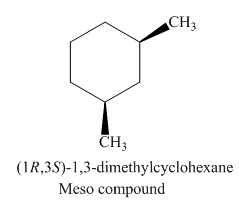
Figure 4
The possible stereoisomers for the given cycloalkane are three. Among the three stereoisomers, the labeling of two enantiomers of the given cycloalkane is
(b)
Interpretation: The possible stereoisomers for the given cycloalkane are to be drawn. The labeling of any enantiomers, diastereomers or meso compound is to be stated.
Concept introduction: A carbon atom that has four nonequivalent atoms or groups attached to it is known as chiral carbon atom. Chiral carbon centers are also called as asymmetric or stereogenic centers.
The stereocenters are carbon atoms on which the interchanging of two atoms or groups results in the formation of new stereoisomers. The stereocenters are also known as stereogenic centers.
Answer to Problem 5.56P
The possible stereoisomers for the given cycloalkane are two. Both are meso compounds and these meso compound are labeled as done as
Explanation of Solution
The given cycloalkane is,

Figure 5
Due to the presence of cyclohexane ring attached with two methyl groups at first and fourth carbon atoms, the IUPAC name of this cycloalkane is
This
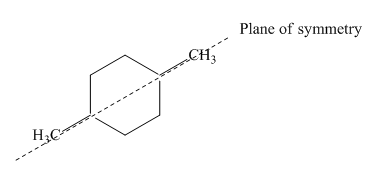
Figure 6
Thus, this compound forms only two stereoisomers one is cis isomer and second is trans isomer. The given compound,
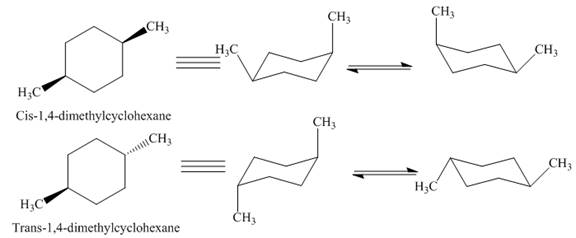
Figure 7
Thus,
The possible stereoisomers for the given cycloalkane are two. Both are meso compounds and these meso compound are labeled as done as
(c)
Interpretation: The possible stereoisomers for the given cycloalkane are to be drawn. The labeling of any enantiomers, diastereomers or meso compound is to be stated.
Concept introduction: A carbon atom that has four nonequivalent atoms or groups attached to it is known as chiral carbon atom. Chiral carbon centers are also called as asymmetric or stereogenic centers.
The stereocenters are carbon atoms on which the interchanging of two atoms or groups results in the formation of new stereoisomers. The stereocenters are also known as stereogenic centers.
Answer to Problem 5.56P
The possible stereoisomers for the given cycloalkane are four. It contains two pairs of enantiomers, the labeling of two pairs of enantiomers of the given cycloalkane is
Explanation of Solution
The given cycloalkane is,
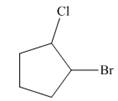
Figure 8
Due to the presence of cyclopentane ring attached with one bromine atom and one chlorine atom at first and second carbon atom respectively, the IUPAC name of this cycloalkane is
Where,
•
Substitute the number of chiral centers of
Thus, the total number of stereoisomers of
The four stereoisomers of
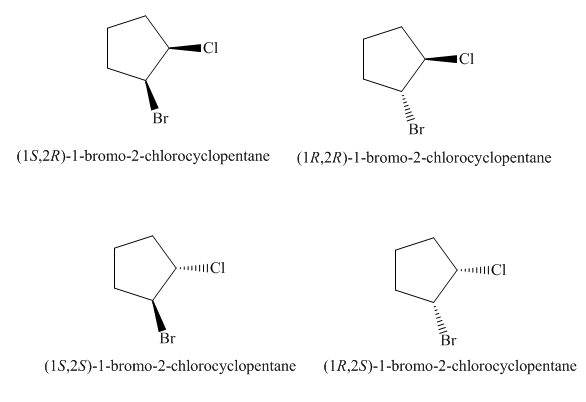
Figure 9
The compound,
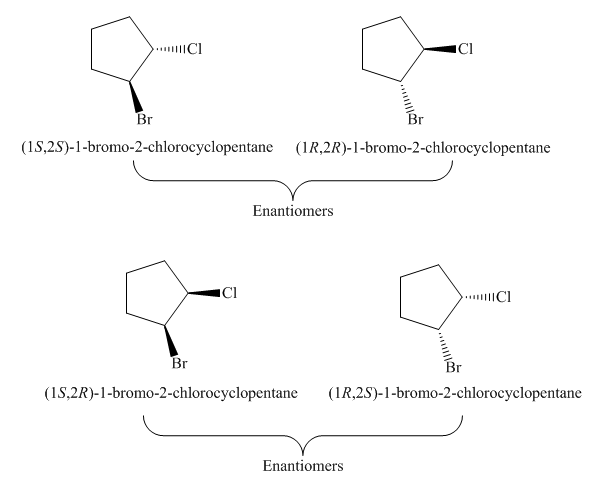
Figure 10
The possible stereoisomers for the given cycloalkane are four. It contains two pairs of enantiomers, the labeling of two pairs of enantiomers of the given cycloalkane is
Want to see more full solutions like this?
Chapter 5 Solutions
Organic Chemistry
- Using the graphs could you help me explain the answers. I assumed that both graphs are proportional to the inverse of time, I think. Could you please help me.arrow_forwardSynthesis of Dibenzalacetone [References] Draw structures for the carbonyl electrophile and enolate nucleophile that react to give the enone below. Question 1 1 pt Question 2 1 pt Question 3 1 pt H Question 4 1 pt Question 5 1 pt Question 6 1 pt Question 7 1pt Question 8 1 pt Progress: 7/8 items Que Feb 24 at You do not have to consider stereochemistry. . Draw the enolate ion in its carbanion form. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. ⚫ Separate multiple reactants using the + sign from the drop-down menu. ? 4arrow_forwardShown below is the mechanism presented for the formation of biasplatin in reference 1 from the Background and Experiment document. The amounts used of each reactant are shown. Either draw or describe a better alternative to this mechanism. (Note that the first step represents two steps combined and the proton loss is not even shown; fixing these is not the desired improvement.) (Hints: The first step is correct, the second step is not; and the amount of the anhydride is in large excess to serve a purpose.)arrow_forward
- Hi I need help on the question provided in the image.arrow_forwardDraw a reasonable mechanism for the following reaction:arrow_forwardDraw the mechanism for the following reaction: CH3 CH3 Et-OH Et Edit the reaction by drawing all steps in the appropriate boxes and connecting them with reaction arrows. Add charges where needed. Electron-flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created. H± EXP. L CONT. י Α [1] осн CH3 а CH3 :Ö Et H 0 N о S 0 Br Et-ÖH | P LL Farrow_forward
- 20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCl. What is the molarity of the HCl?arrow_forward20.00 mL of 0.025 M HCl is titrated with 0.035 M KOH. What volume of KOH is needed?arrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCl. What is the molarity of the HCl?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

