
5.50.19 In chemical vapor deposition (CVD), a semiconducting or insulating solid material is formed in a reaction between a gaseous species and a species adsorbed on the surface of silicon wafers (disks about 10 cm in diameter and l mm thick). The coated wafers are subjected to further processing to produce the microelectronic chips in computers and most other electronic devices in use today.
In one such process, silicon dioxide (MW = 60.06, SG = 2.67) is formed in the reaction between gaseous dichlorosilane (DCS) and adsorbed nitrous oxide:
A mixture of DCS and N2O ?ows through a “boat reactor"—a horizontal pipe in which 50 to 100 silicon wafers about 12 cm in diameter and 1 mm thick are set upright along the reactor length, with about 20 mm separation between each wafer. A side view of the reactor is shown below:
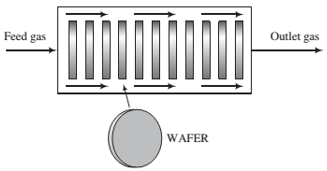
The feed gas enters the reactor at a rate of 3.74 SCMM (standard cubic meters per minute) and contains 22.0 mole% DCS and the balance N2O. In the reactor, the gas ?ows around the wafers, DCS and N2O diffuse into the spaces between the wafers, N2O is adsorbed on the wafer surfaces, and the adsorbed N2O reacts with gaseous DCS. The silicon dioxide formed remains on the surface, and the nitrogen and hydrogen chloride go into the gas phase and eventually leave the reactor with the unconsumed reactants. The temperature and absolute pressure in the reactor are constant at 900°C and 604 millitorr.
(a) The percentage conversion of DCS at a certain axial position (distance along the length of the reactor) is 60%. Calculate the volumetric ?ow rate (m3/min) of gas at this axial position.
(b) The rate of deposition of silicon dioxide per unit area of wafer surface is given by the formula
where
(c) Consider a wafer located at the axial position determined in Part (b). How thick is the silicon dioxide layer on that wafer after two hours of reactor operation, assuming that gas diffusion is rapid enough at the low reactor pressure for the composition of the gas (and hence the component partial pressures) to be uniform over the wafer surface? Express your answer in angstroms, where
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
EBK ELEMENTARY PRINCIPLES OF CHEMICAL P
Additional Science Textbook Solutions
Java: An Introduction to Problem Solving and Programming (8th Edition)
Electric Circuits. (11th Edition)
Elementary Surveying: An Introduction To Geomatics (15th Edition)
Management Information Systems: Managing The Digital Firm (16th Edition)
Modern Database Management
Starting Out with Java: From Control Structures through Objects (7th Edition) (What's New in Computer Science)
- =Naming benzene derivatives Name these organic compounds: structure C1 CH3 name ☐ CH3 ப C1 × ☐arrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see image **NOTE: The compound on the left is the starting point, and the compound on the right is the final product. Please show the steps in between to get from start to final, please. These are not two different compounds that need to be worked.arrow_forwardI dont understand this.arrow_forward
- Can you please explain this prooblem to me, show me how the conjugation is added, did I add them in the correct places and if so please show me. Thanks!arrow_forwardBasic strength of organic bases.arrow_forwardNucleophilic Aromatic Substitution: What is the product of the reaction? What is the name of the intermediate complex? *See imagearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





