
(a)
Interpretation:
The graph of diffusion coefficient (D) m2/s, v/s inverse of temperature (1/T) needs to be plotted using the given data.
Concept Introduction:
Diffusion coefficient (D) is given by the following relation:
Where,
Answer to Problem 5.44P
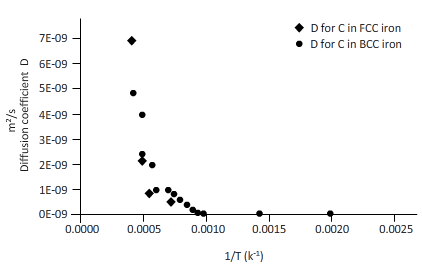
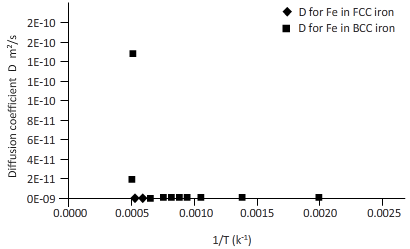
The curves between diffusion coefficient and inverse temperature for Fe atom in FCC and BCC iron are as follows:
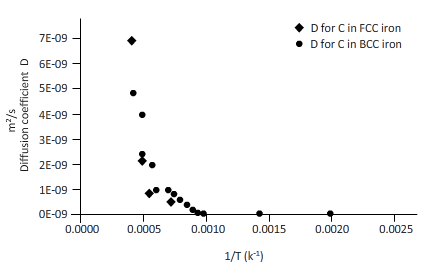
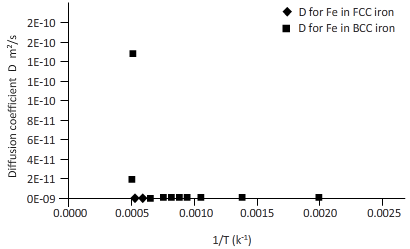
Explanation of Solution
Given Information:
| Diffusion couple | Diffusion | Q(J/mol) | Do(m2/s) |
| C in FCC iron | Interstitial | ||
| C in BCC iron | Interstitial | ||
| Fe in FCC iron | vacancy | ||
| Fe in BCC iron | vacancy |
Calculation:
The diffusion coefficient can be calculated as follows:
Here,
Equation (1) becomes
Hence, coefficient of diffusion for C in FCC iron at
Now, draw a table to calculate the coefficient of diffusion for C in FCC iron and for C in BCC iron diffusion temperature.
| T(k) | 1/T(k-1) | D for C in FCC iron ( | D for C in BCC iron ( |
| 2000 | 0.0005 | 6E-09 | 6E-09 |
| 1750 | 0.0006 | 2E-09 | 3E-09 |
| 1500 | 0.0007 | 4E-10 | 1E-09 |
| 1250 | 0.0008 | 4E-11 | 2E-10 |
| 1200 | 0.0008 | 2E-11 | 2E-10 |
| 1150 | 0.0009 | 1E-11 | 1E-10 |
| 1100 | 0.0009 | 7E-12 | 8E-11 |
| 1000 | 0.0010 | 1E-12 | 3E-11 |
| 750 | 0.0013 | 6E-15 | 9E-13 |
| 500 | 0.0020 | 9E-20 | 8E-16 |
From the table, plot a curve between inverse temperature (1/T) and diffusion coefficient D.
Again draw a table to calculate the coefficient of diffusion for Fe in FCC iron and for Fe in BCC iron at different temperature.
| T(k) | 1/k(k-1) | D for C in FCC iron ( | D for C in BCC iron ( |
| 2000 | 0.0005 | 3E-12 | 2E-10 |
| 1750 | 0.0006 | 3E-13 | 2E-11 |
| 1500 | 0.0007 | 1E-14 | 1E-12 |
| 1250 | 0.0008 | 1E-16 | 2E-14 |
| 1200 | 0.0008 | 5E-17 | 8E-15 |
| 1150 | 0.0009 | 1E-17 | 3E-15 |
| 1100 | 0.0009 | 4E-18 | 9E-16 |
| 1000 | 0.0010 | 2E-19 | 6E-17 |
| 750 | 0.0013 | 2E-24 | 3E-21 |
| 500 | 0.0020 | 5E-34 | 9E-30 |
Plotting the curve between inverse temperature (1/T) and diffusion coefficient (D) for Fe atom in FCC and BCC.
(b)
Interpretation:
The temperature range at which the iron-transition takes place needs to be determined from the graphs.
Concept Introduction:
Iron transition is the period in which a subject change from one state to another takes place.
Answer to Problem 5.44P
The iron transition for BCC to FCC takes place in a range of temperature 1150 K-1200 K.
The iron transition for FCC to BCC takes place in the range of 1600 K-1700 K.
Explanation of Solution
Given Information:
| Diffusion couple | Diffusion mechanism | Q(J/mol) | Do(m2/s) |
| C in FCC iron | Interstitial | ||
| C in BCC iron | Interstitial | ||
| Fe in FCC iron | vacancy | ||
| Fe in BCC iron | vacancy |
The curves between diffusion coefficient and inverse temperature for Fe atom in FCC and BCC iron are as follows:
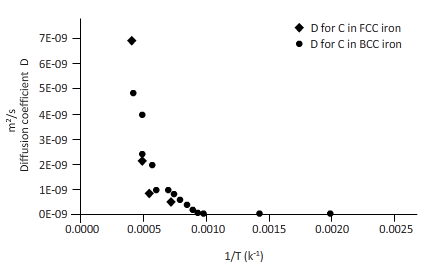
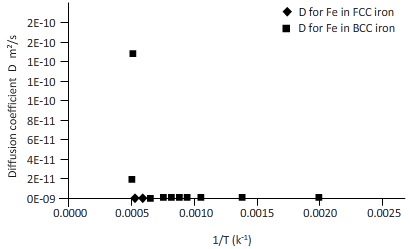
From the curve (1),
BCC iron to FCC iron transition takes place in a range of temperature 1150 K-1200 K.
From the curve (2),the iron transition from BCC to FCC iron take place in the temperature range 1600 K-1700 K.
(c)
Interpretation:
The reason for the higher activation energy for diffusion in FCC iron than carbon needs to be explained.
Concept Introduction:
Activation energy, D is related to the size of the atom.
Answer to Problem 5.44P
An atomic number of carbon (C) is 6 while the atomic number of iron (Fe) is 26.
As we know that activation is related to the size of atom hence more atomic number greater is the activation energy required.
Explanation of Solution
Diffusion atom is placed in a vacancy position.
But in some atoms, there is a defect due to more interstitial position and less vacancy. Diffusion atom takes less time to reach in interstitial position than vacancy. Activation energy co-relates with atomic number. Hence, more atomic number, maximum activation energy is required. The atomic number of carbon is 4 and that of iron is 26. Thus, the activation energy for diffusion of Fe is higher than that of C.
(d)
Interpretation:
The reason for the higher activation energy for the diffusion of FCC than BCC iron needs to be explained.
Concept Introduction:
Activation energy is directly proportional to the packing fraction. Packing fraction is a bond formed by atoms.
Answer to Problem 5.44P
Packing fraction of FCC structure is 0.71 while that for the BCC structure is 0.68.
Hence, the activation energy required for FCC is more than BCC.
Explanation of Solution
Packing fraction is the measurement of loss or gain of the total mass in a group of the nucleus which directly relates atomic bond. Packing fraction is correlated with activation energy. The packing fraction for FCC structure is 0.74 and that for the BCC structure is 0.68.
The activation energy for diffusion higher in FCC as compared to the BCC iron.
(e)
Interpretation:
Whether the diffusion rate in FCC is faster than in BCC or not needs to be determined.
Concept Introduction:
Diffusion coefficient (D) is given by the following relation:
Where,
Answer to Problem 5.44P
Diffusion rate in FCC structure is slower than the BCC structure.
Explanation of Solution
Taking T= 1175 K from temperature range of BCC to FCC iron transition.
Since,
Substitute,
Equation (1) becomes,
Again to find out 'D' in BCC iron for carbon.
Put
Equation (1) becomes,
From (a) and (b), one can say that the diffusion coefficient is more in BCC structure than the FCC.
Hence, diffusion atoms move faster in BCC structure compared to FCC structure.
Want to see more full solutions like this?
Chapter 5 Solutions
Essentials Of Materials Science And Engineering
- 2. Consider the following pseudocode for partition: function partition (A,L,R) pivotkey = A [R] t = L for i L to R-1 inclusive: if A[i] A[i] t = t + 1 end if end for A [t] A[R] return t end function Suppose we call partition (A,0,5) on A=[10,1,9,2,8,5]. Show the state of the list at the indicated instances. Initial A After i=0 ends After 1 ends After i 2 ends After i = 3 ends After i = 4 ends After final swap 10 19 285 [12 pts]arrow_forwardDraw as a 3D object/Isometricarrow_forwardpower systemsarrow_forward
- .NET Interactive Solving Sudoku using Grover's Algorithm We will now solve a simple problem using Grover's algorithm, for which we do not necessarily know the solution beforehand. Our problem is a 2x2 binary sudoku, which in our case has two simple rules: •No column may contain the same value twice •No row may contain the same value twice If we assign each square in our sudoku to a variable like so: 1 V V₁ V3 V2 we want our circuit to output a solution to this sudoku. Note that, while this approach of using Grover's algorithm to solve this problem is not practical (you can probably find the solution in your head!), the purpose of this example is to demonstrate the conversion of classical decision problems into oracles for Grover's algorithm. Turning the Problem into a Circuit We want to create an oracle that will help us solve this problem, and we will start by creating a circuit that identifies a correct solution, we simply need to create a classical function on a quantum circuit that…arrow_forwardpower systemsarrow_forwardPost-tensioned AASHTO Type II girders are to be used to support a deck with unsupported span equal to 10 meters. Two levels of Grade 250, 10 x 15.2 mm Ø 7-wire strand are used to tension the girders with 5 tendons per level, where the tendons on top stressed before the ones on the bottom. The girder is simply supported at both ends. The anchors are located 100 mm above the neutral axis at the supports while the eccentricity is measured at 400 mm at the midspan. The tendon profile follows a parabolic shape using a rigid metal sheathing. A concrete topping (slab) 130 mm thick is placed above the beam with a total tributary width of 4 meters. Use maximum values for ranges (table values). Assume that the critical section of the beam is at 0.45LDetermine the losses (friction loss, anchorage, elastic shortening, creep, shrinkage, relaxation). Determine the stresses at the top fibers @ critical section before placing a concrete topping, right after stress transfer. Determine the stress at the…arrow_forward
- A random poly(styrene-butadiene) copoly- mer has a number-average molecular weight of 350,000 g/mol and a degree of polymerization of 5000. Compute the fraction of styrene and buta- diene repeat units in this copolymer. H H | | -C-C- 방 Harrow_forwardDesign and assemble on the fluidsim (or a draft) the Hydraulic Drive Circuit, with the following characteristics: (a) Sequential operation, pressure, for the advance and return of the cylinders (according to the proper operation for the device) controlled by a directional 4x3 way, closed center; (b) Speed control for the cylinders, according to the load signal; (c) Pressure counterbalance for cylinder A, in order to compensate for the weight of the assembly.arrow_forwardThis is an old exam practice question. The answer is Pmax = 218.8 kN normal stress governs but why?arrow_forward
 MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc
MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage,
Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage, Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning
Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION
Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON
Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY
Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY





