
Concept explainers
(a)
Interpretation:
Considering a gas container equipped with a movable piston, the change in volume and pressure should be explained.
Concept Introduction:
Ideal gas equation:
At a constant temperature (K) and pressure (P), the volume (v) occupied by the no of moles of any gas is known as ideal gas equation.
Ideal gas equation:
And the SI units are
(a)
Answer to Problem 5.31QP
An increase of pressure by 2 times to the original, will decrease volume by
Explanation of Solution
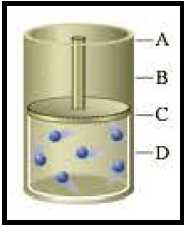
Figure 1
From
Pressure and volume are inversely proportional to each other. So the increase in pressure causes decrease in volume C to D.
The change in volume with respect to pressure was explained.
(b)
Interpretation:
Considering a gas container equipped with a movable piston, the change in volume and pressure should be explained.
Concept Introduction:
Ideal gas equation:
At a constant temperature (K) and pressure (P), the volume (v) occupied by the no of moles of any gas is known as ideal gas equation.
Ideal gas equation:
And the SI units are
(b)
Answer to Problem 5.31QP
An increase of volume (C to A) by 2 times to the original, will decrease pressure by
Explanation of Solution

Figure 1
From Ideal
Pressure and volume are inversely proportional to each other. So the increase in volume (C to A) causes decrease in pressure as molecules get more space to move around.
The change in volume with respect to pressure was explained.
(c)
Interpretation:
Considering a gas container equipped with a movable piston, the change in temperature when volume changes from C to B should be explained.
Concept Introduction:
Ideal gas equation:
At a constant temperature (K) and pressure (P), the volume (v) occupied by the no of moles of any gas is known as ideal gas equation.
Ideal gas equation:
And the SI units are
(c)
Answer to Problem 5.31QP
The change in kelvin temperature by 1.5 factor would cause the change in volume (C to B)
Explanation of Solution

Figure 1
From Ideal gas law,
Temperature and volume are proportional to each other. So the increase in volume (C to A) causes increase in temperature.
The change in volume with respect to temperature was explained
(d)
Interpretation:
The change in volume and pressure when the number of moles increased in a container with a moving piston should be explained.
Concept Introduction:
Ideal gas equation:
At a constant temperature (K) and pressure (P), the volume (v) occupied by the no of moles of any gas is known as ideal gas equation.
Ideal gas equation:
And the SI units are
(d)
Answer to Problem 5.31QP
The increase in the number of moles by 2 factor would cause the increase in volume by 2 factor (C to A).
Explanation of Solution

Figure 1
From Ideal gas law,
Number of particles and volume are proportional to each other. So the increase in molecules would increase the volume by 2 factor (C to A). Since the piston is movable the pressure will not be affected (similar to starting pressure) even though number of particles and pressure are directly proportional to each other.
The change in volume and pressure when the number of moles increased in a container with a moving piston was explained.
Want to see more full solutions like this?
Chapter 5 Solutions
Student Solutions Manual for Ebbing/Gammon's General Chemistry
- A gas following mole compositions at 120 \deg F, 13.8 psia. N2% 2, CH 4% 79C2H6 % 19. Volume fractionn?arrow_forwardPlease correct answer and don't used hand raitingarrow_forwardOrder-disorder phenomenaa) do not have conductive properties.b) are cooperative.c) have few industrial implications.arrow_forward
- Unshared, or lone, electron pairs play an important role in determining the chemical and physical properties of organic compounds. Thus, it is important to know which atoms carry unshared pairs. Use the structural formulas below to determine the number of unshared pairs at each designated atom. Be sure your answers are consistent with the formal charges on the formulas. CH. H₂ fo H2 H The number of unshared pairs at atom a is The number of unshared pairs at atom b is The number of unshared pairs at atom c is HC HC HC CH The number of unshared pairs at atom a is The number of unshared pairs at atom b is The number of unshared pairs at atom c isarrow_forwardDraw curved arrows for the following reaction step. Arrow-pushing Instructions CH3 CH3 H H-O-H +/ H3C-C+ H3C-C-0: CH3 CH3 Harrow_forward1:14 PM Fri 20 Dec 67% Grade 7 CBE 03/12/2024 (OOW_7D 2024-25 Ms Sunita Harikesh) Activity Hi, Nimish. When you submit this form, the owner will see your name and email address. Teams Assignments * Required Camera Calendar Files ... More Skill: Advanced or complex data representation or interpretation. Vidya lit a candle and covered it with a glass. The candle burned for some time and then went off. She wanted to check whether the length of the candle would affect the time for which it burns. She performed the experiment again after changing something. Which of these would be the correct experimental setup for her to use? * (1 Point) She wanted to check whether the length of the candle would affect the time for which it burns. She performed the experiment again after changing something. Which of these would be the correct experimental setup for her to use? A Longer candle; No glass C B Longer candle; Longer glass D D B Longer candle; Same glass Same candle; Longer glassarrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning





