
Concept explainers
(a)
Interpretation:
Whether the molecules in the given pair are superimposable or nonsuperimposable is to be determined.
Concept introduction:
Molecules are nonsuperimposable if there is no orientation in which all atoms of both molecules can be lined up perfectly (i.e., superimposed). Every molecule can have a mirror image. But not every molecule can have a nonsuperimposable mirror image.
Answer to Problem 5.1P
The given two molecules are superimposable.
Explanation of Solution
Molecules in the given pair are
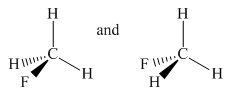
In the above pair, both molecules have carbon as the central atom with one fluorine atom and three hydrogen atoms as an attachment. In the left molecule, fluorine is pointing towards the observer and one hydrogen atom is pointing away from the observer while in the second molecule fluorine is pointing away from the observer and one hydrogen atom is pointing towards the observer.
To check the molecule in different orientation the molecule present at right is rotated.
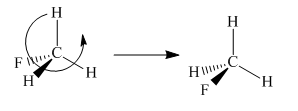
The molecule after the rotation has the exact similar orientation of the atoms as the left molecule in the given pair i.e. all atoms are lined up perfectly.
Thus both the molecules in the given pair are superimposable.
All atoms of both molecules are lined perfectly hence superimposable.
(b)
Interpretation:
Whether the molecules in the given pair are superimposable or nonsuperimposable is to be determined.
Concept introduction:
Molecules are nonsuperimposable if there is no orientation in which all atoms of both molecules can be lined up perfectly (i.e., superimposed). Every molecule can have a mirror image, but not every molecule can have a nonsuperimposable mirror image.
Answer to Problem 5.1P
The given two molecules are superimposable.
Explanation of Solution
Molecules in the given pair are
In given pair, both molecules are mirror images of each other. If the molecule at right is rotated, it gives the molecule exactly similar to the left one.
Thus both the molecules in the given pair are superimposable.
All atoms of both the molecules are lined perfectly hence superimposable.
(c)
Interpretation:
Whether the molecules in the given pair are superimposable or nonsuperimposable is to be determined.
Concept introduction:
Molecules are nonsuperimposable if there is no orientation in which all atoms of both molecules can be lined up perfectly (i.e., superimposed). Every molecule can have a mirror image, but not every molecule can have a nonsuperimposable mirror image.
Answer to Problem 5.1P
The given two molecules are nonsuperimposable.
Explanation of Solution
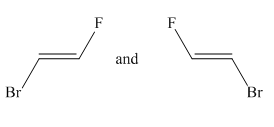
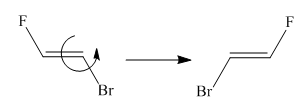
Molecules in the given pair are:
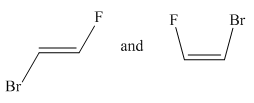
Two molecules given in the pair are the cis-trans isomers of the same molecule. To get the trans isomer from cis, rotation is needed around the double bond which is restricted. Hence the atoms in both molecules are not lined up perfectly, thus the given two molecules are nonsuperimposable.
All atoms of both molecules are not lined perfectly hence nonsuperimposable.
(d)
Interpretation:
Whether the molecules in the given pair are superimposable or nonsuperimposable is to be determined.
Concept introduction:
The molecules are nonsuperimposable if there is no orientation in which all atoms of both molecules can be lined up perfectly (i.e., superimposed). Every molecule can have a mirror image, but not every molecule can have a nonsuperimposable mirror image.
Answer to Problem 5.1P
The given two molecules are superimposable.
Explanation of Solution
Molecules in the given pair are:
![]()
Two molecules given in pairs are the mirror images of each other. If one of the two given molecules is rotated around the central carbon atom it gives the exact similar molecule as another.
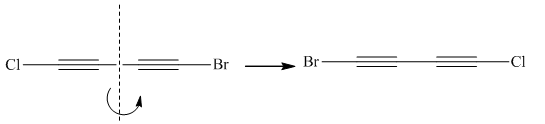
Rotation of the molecule gives the molecule with a similar orientation as another molecule, thus two molecules are superimposable.
All atoms of both molecules are lined perfectly, hence superimposable.
(e)
Interpretation:
Whether the molecules in the given pair are superimposable or nonsuperimposable is to be determined.
Concept introduction:
The molecules are nonsuperimposable if there is no orientation in which all atoms of both molecules can be lined up perfectly (i.e., superimposed). Every molecule can have a mirror image, but not every molecule can have a nonsuperimposable mirror image.
Answer to Problem 5.1P
The given two molecules are superimposable.
Explanation of Solution
Molecules in the given pair are:
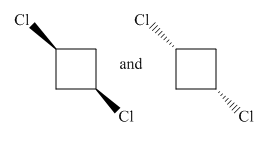
Both molecules in the given pair are isomers of the same molecules thus have the same attachments of the molecule with different orientations. Two chlorine atoms in the left molecule are pointing towards the observer while two chlorine atoms in the right molecule are pointing away from the observer. If one of the molecule rotates with
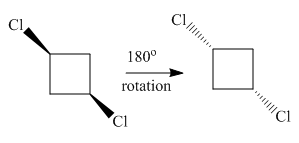
The molecule after the rotation of the left molecule has the exact similar orientation as the molecule at right, all atoms of both molecules are lined perfectly representing the molecules in the given pair are superimposable.
All atoms of both molecules are lined perfectly hence superimposable.
(f)
Interpretation:
Whether the molecules in the given pair are superimposable or nonsuperimposable is to be determined.
Concept introduction:
Molecules are nonsuperimposable if there is no orientation in which all atoms of both molecules can be lined up perfectly (i.e., superimposed). Every molecule can have a mirror image, but not every molecule can have a nonsuperimposable mirror image.
Answer to Problem 5.1P
The given two molecules are nonsuperimposable.
Explanation of Solution
Molecules in the given pair are
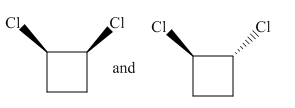
In the molecule at the left, both chlorine atoms are pointing towards the observer while in the molecule at right one chlorine atom is pointing towards the observer and one is pointing away from the observer. To get a similar orientation, rotation of the atom with the ring is needed which is restricted. Thus there is no orientation of both molecules lined perfectly, representing the molecules in the given pair are nonsuperimposable.
All atoms of both molecules are not lined perfectly, hence nonsuperimposable.
(g)
Interpretation:
Whether the molecules in the given pair are superimposable or nonsuperimposable is to be determined.
Concept introduction:
Molecules are nonsuperimposable if there is no orientation in which all atoms of both molecules can be lined up perfectly (i.e., superimposed). Every molecule can have a mirror image. But not every molecule can have a nonsuperimposable mirror image.
Answer to Problem 5.1P
The given two molecules are superimposable.
Explanation of Solution
Molecules in the given pair are
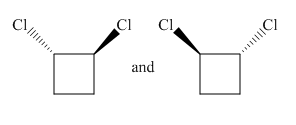
In the given pair both molecules are mirror images of each other having one chlorine atom pointing towards the observer and another pointing away from the observer.
The complete rotation of
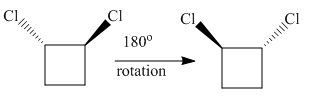
The molecule after the rotation of the left molecule has the exact similar orientation as the molecule at right, all atoms of both molecules are lined perfectly representing the molecules in the given pair are superimposable.
All atoms of both molecules are lined perfectly hence superimposable.
(h)
Interpretation:
Whether the molecules in the given pair are superimposable or nonsuperimposable is to be determined.
Concept introduction:
The molecules are nonsuperimposable if there is no orientation in which all atoms of both molecules can be lined up perfectly (i.e., superimposed). Every molecule can have a mirror image. But not every molecule can have a nonsuperimposable mirror image.
Answer to Problem 5.1P
The given two molecules are nonsuperimposable.
Explanation of Solution
Molecules in the given pair are
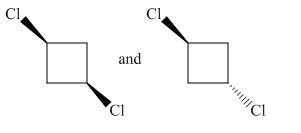
In the molecule at left both chlorine atoms are pointing towards the observer while in the molecule at right one chlorine atom is pointing towards the observer and one is pointing away from the observer. To get a similar orientation, rotation of the atom with the ring is needed which is restricted. Thus no orientation of both molecules lined perfectly, representing the molecules in the given pair are nonsuperimposable.
All atoms of both molecules are not lined perfectly hence nonsuperimposable.
Want to see more full solutions like this?
Chapter 5 Solutions
EBK ORGANIC CHEMISTRY: PRINCIPLES AND M
- Draw the major product of the Claisen condensation reaction between two molecules of this ester. Ignore inorganic byproducts. Incorrect, 5 attempts remaining 1. NaOCH3/CH3OH 2. Acidic workup Select to Draw O Incorrect, 5 attempts remaining The total number of carbons in the parent chain is incorrect. Review the reaction conditions including starting materials and/or intermediate structures and recount the number of carbon atoms in the parent chain of your structure. OKarrow_forwardUsing a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c d) Calculate x for the 1645 cm-1 bandarrow_forwardConvert 1.38 eV into wavelength (nm) and wavenumber (cm-1) (c = 2.998 x 108 m/s; h = 6.626 x 10-34 J*s).arrow_forward
- Can you help me understand the CBC method on metal bridging by looking at this problem?arrow_forwardA partir de Aluminio y Co(NO3)2ꞏ6H2O, indicar las reacciones a realizar para obtener Azul de Thenard (Al2CoO4).arrow_forwardTo obtain Thenard Blue (Al2CoO4), the following reaction is correct (performed in an oven):Al(OH)3 + Co(OH)2 → Al2CoO4 + 4 H2Oarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
