
Concept explainers
(a)
Interpretation:
The condensed structural formula and systematic name should be given for the molecular formula of
Concept introduction:
A condensed structural formula is a system of writing organic structures in a line of text.
Isomer: A molecule having the same molecular formula but with different chemical structure is called isomer.
Constitutional Isomers: A molecule having same molecular formula with different structural formulas (Difference in the connectivity of the molecule is called constitutional isomer).
Any organic molecule can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry).IUPAC name consists of three parts, namely Prefix, suffix and root word.
Prefix- Represents the substituent present in the molecule and its position in the root name.
Suffix- Denotes the presence of
Root word - Represents the longest continuous carbon skeleton of the organic molecule.
Alkenes are a class of hydrocarbons. The carbon-carbon double bond is called as alkenes and it is also called as olefins.
(b)
Interpretation:
The E and Z isomer of the alkene has to be identified.
Concept introduction:
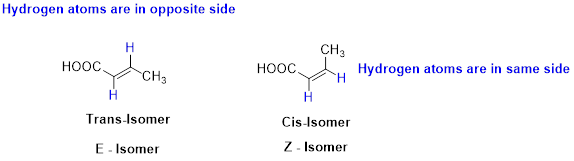
E and Z isomerism:
The two similar groups (or higher priority groups) are in same side in double bond of alkenes is called as cis isomer (or Z-isomer). Two similar groups (or higher priority groups) are opposite side in double bond of alkenes is called as trans isomer (or E-isomer).
Example:
(c)
Interpretation:
The most stable alkene has to be identified.
Concept Introduction:
Stability of alkene:
Stability of alkene depends on the following factors,
The smallest heat hydrogenation of alkene is more stable. The number of hydrogens bonded to its
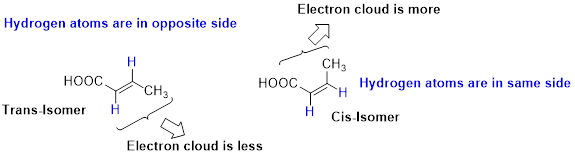
Stability of Cis–trans alkene:
The two similar groups (or higher priority groups) are in same side in double bond of alkenes is called as cis isomer (or Z-isomer). Two similar groups (or higher priority groups) are opposite side in double bond of alkenes is called as trans isomer (or E-isomer).
In cis alkene, molecule are close to each other, hence electron clouds interfere each other therefore strain in the molecule so cis alkene is less stable. Whereas molecule is away from each other, hence electron clouds are will not interfere each other therefore less strain in the molecule, so trans alkene is stable.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Essential Organic Chemistry (3rd Edition)
- I need help with the followingarrow_forwardFor Raman spectroscopy/imaging, which statement is not true regarding its disadvantages? a) Limited spatial resolution. b) Short integration time. c) A one-dimensional technique. d) Weak signal, only 1 in 108 incident photons is Raman scattered. e) Fluorescence interference.arrow_forwardUsing a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c. (Please provide a full derivation of the equation for x from the equation for I). d) Calculate x for the 1645 cm-1 bandarrow_forward
- For CARS, which statement is not true regarding its advantages? a) Contrast signal based on vibrational characteristics, no need for fluorescent tagging. b) Stronger signals than spontaneous Raman. c) Suffers from fluorescence interference, because CARS signal is at high frequency. d) Faster, more efficient imaging for real-time analysis. e) Higher resolution than spontaneous Raman microscopy.arrow_forwardDraw the major product of the Claisen condensation reaction between two molecules of this ester. Ignore inorganic byproducts. Incorrect, 5 attempts remaining 1. NaOCH3/CH3OH 2. Acidic workup Select to Draw O Incorrect, 5 attempts remaining The total number of carbons in the parent chain is incorrect. Review the reaction conditions including starting materials and/or intermediate structures and recount the number of carbon atoms in the parent chain of your structure. OKarrow_forwardUsing a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c d) Calculate x for the 1645 cm-1 bandarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning


