
Concept explainers
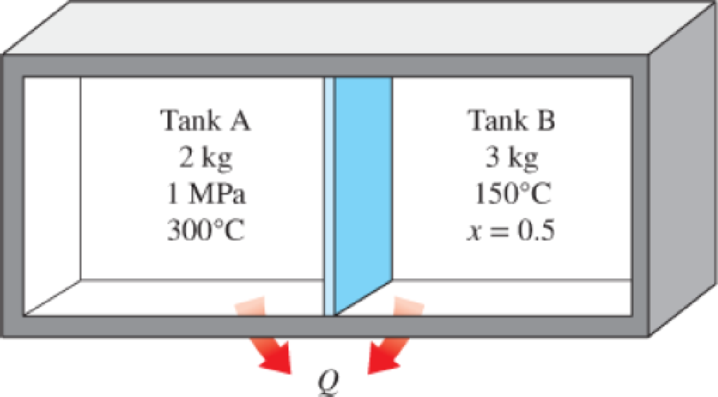
Two tanks (Tank A and Tank B) are separated by a partition. Initially Tank A contains 2 kg of steam at 1 MPa and 300°C while Tank B contains 3 kg of saturated liquid–vapor mixture at 150°C with a vapor mass fraction of 50 percent. The partition is now removed and the two sides are allowed to mix until
FIGURE P4–44
(a)
The final temperature of the steam for a tank.
The quality of the steam at the final state for a tank.
Answer to Problem 44P
The final temperature of the steam is
The quality of the steam at the final state is
Explanation of Solution
Write the expression for the energy balance equation.
Here, the total energy entering the system is
Simplify Equation (I) and write energy balance relation of two tanks.
Here, the boundary work to be done into the system is
Substitute 0 for
Here, the initial mass of tank A is
Write the expression for volume of tank A.
Here, the volume of the tank A is
Write the expression for volume of tank B.
Here, the volume of the tank B is
Write the expression for total mass of tank.
Write the expression for total volume contained in both tanks.
Write the expression for final specific volume of tank.
Conclusion:
Convert the unit of pressure from kPa to MPa.
From the Table A-6, “Superheated water”, obtain the value of initial specific volume
From the Table A-4, “Superheated water”, obtain the value of initial specific volume and specific internal energy for tank B.
At the initial temperature and quality of the steam at the final state of tank B as 150 C and 0.50.
Determine the specific volume of a two-phase system for tank B.
Here, the specific volume of saturated liquid for tank B is
Here, the specific internal energy of saturated liquid for tank B is
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Refer Table A-5, “Saturated water-pressure”, obtain the final temperature of the steam.
At final pressure of the steam is
Thus, the final temperature of the steam is
From the Table A-5, “Saturated water-pressure”, obtain the value of quality of the steam at the final state and final specific internal energy for tank B.
At the final pressure and specific volume at the final state as 300 kPa and
Determine the specific volume of a two-phase system for steam.
Here, the specific volume of saturated liquid for steam is
Here, the specific internal energy of saturated liquid for steam is
Substitute
Thus, the quality of the steam at the final state is
Substitute
(b)
The amount of heat lost from the tanks.
Answer to Problem 44P
The amount of heat lost from the tanks is
Explanation of Solution
Conclusion:
Substitute
Thus, the amount of heat lost from the tanks is
Want to see more full solutions like this?
Chapter 4 Solutions
THERMODYNAMICS LLF W/ CONNECT ACCESS
- A mass of ideal gas in a closed piston-cylinder system expands from 427 °C and 16 bar following the process law, pv1.36 = Constant (p times v to the power of 1.36 equals to a constant). For the gas, initial : final pressure ratio is 4:1 and the initial gas volume is 0.14 m³. The specific heat of the gas at constant pressure, Cp = 0.987 kJ/kg-K and the specific gas constant, R = 0.267 kJ/kg.K. Determine the change in total internal energy in the gas during the expansion. Enter your numerical answer in the answer box below in KILO JOULES (not in Joules) but do not enter the units. (There is no expected number of decimal points or significant figures).arrow_forwardmy ID# 016948724. Please solve this problem step by steparrow_forwardMy ID# 016948724 please find the forces for Fx=0: fy=0: fz=0: please help me to solve this problem step by steparrow_forward
- My ID# 016948724 please solve the proble step by step find the forces fx=o: fy=0; fz=0; and find shear moment and the bending moment diagran please draw the diagram for the shear and bending momentarrow_forwardMy ID#016948724. Please help me to find the moment of inertia lx ly are a please show to solve step by stepsarrow_forwardplease solve this problem step by steparrow_forward
- Please do not use any AI tools to solve this question. I need a fully manual, step-by-step solution with clear explanations, as if it were done by a human tutor. No AI-generated responses, please.arrow_forwardPlease do not use any AI tools to solve this question. I need a fully manual, step-by-step solution with clear explanations, as if it were done by a human tutor. No AI-generated responses, please.arrow_forwardPlease do not use any AI tools to solve this question. I need a fully manual, step-by-step solution with clear explanations, as if it were done by a human tutor. No AI-generated responses, please.arrow_forward
 Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning
Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning International Edition---engineering Mechanics: St...Mechanical EngineeringISBN:9781305501607Author:Andrew Pytel And Jaan KiusalaasPublisher:CENGAGE L
International Edition---engineering Mechanics: St...Mechanical EngineeringISBN:9781305501607Author:Andrew Pytel And Jaan KiusalaasPublisher:CENGAGE L Automotive Technology: A Systems Approach (MindTa...Mechanical EngineeringISBN:9781133612315Author:Jack Erjavec, Rob ThompsonPublisher:Cengage Learning
Automotive Technology: A Systems Approach (MindTa...Mechanical EngineeringISBN:9781133612315Author:Jack Erjavec, Rob ThompsonPublisher:Cengage Learning



