
The ingenious Stirling engine is a true
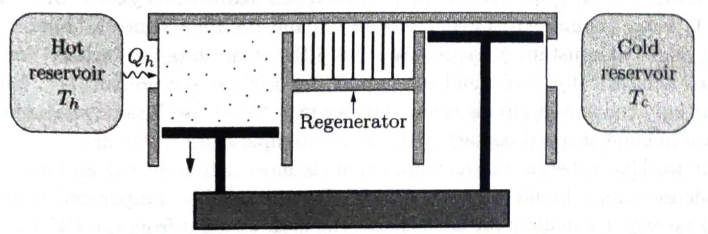
Figure 4.7. A Stirling engine, shown during the power stroke when the hot piston is moving outward and the cold piston is at rest. (For simplicity, the linkages between the two pistons are not shown.)
Trending nowThis is a popular solution!

Chapter 4 Solutions
An Introduction to Thermal Physics
Additional Science Textbook Solutions
Microbiology with Diseases by Body System (5th Edition)
Campbell Essential Biology (7th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Organic Chemistry (8th Edition)
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
- 4a Which of the following values COULD NOT be a magnitude? Choose all that apply. 626 0 -0.806 8.63 -48.5 72 131 156 4b Px = -1248 & Py = 261. Determine P.P = Qx = -1540 & Qy = 375. Determine Q.Q = 4c. T = 1105 & Ty = 425. Determine the two possible values for Tx. 4d. Uy = -38. Which of the following COULD NOT be the value of U? Choose all that apply. 10 70 72 31 47 0 75 38 4e. R has a magnitude of 165. Which of the following COULD be Rx? Choose all that apply. 165 -171 155 0 -156 -165 172 -130arrow_forward9.arrow_forward10.arrow_forward

 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





