
Concept explainers
(a)
Interpretation: To determine the type of reaction whether it will be an
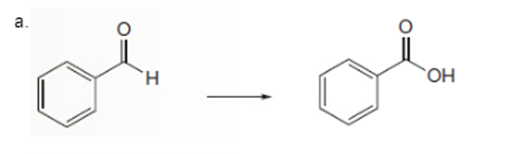
Concept Introduction: Oxidation, which refers to the loss of electrons, is the increase in the oxidation state of its component atoms. When an atom obtains electrons or has its oxidation state reduced, the reduction can occur.
(b)
Interpretation: To determine the type of reaction whether it will be an oxidation or reduction reaction.
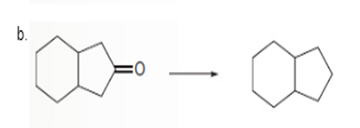
Concept Introduction:
Oxidation, which refers to the loss of electrons, is the increase in the oxidation state of its component atoms. When an atom obtains electrons or has its oxidation state reduced, the reduction can occur.
(c)
Interpretation: To determine the type of reaction whether it will be an oxidation or reduction reaction.
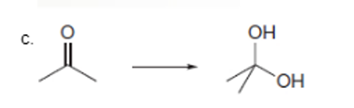
Concept Introduction: Oxidation, which refers to the loss of electrons, is the increase in the oxidation state of its component atoms. When an atom obtains electrons or has its oxidation state reduced, the reduction can occur.
(d)
Interpretation: To determine the type of reaction whether it will be an oxidation or reduction reaction.
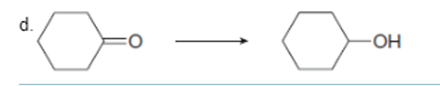
Concept Introduction: Oxidation, which refers to the loss of electrons, is the increase in the oxidation state of its component atoms. When an atom obtains electrons or has its oxidation state reduced, the reduction can occur.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
ORGANIC CHEMISTRY - LOOSELEAF W/CONNECT
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

