
ORGANIC CHEMISTRY - LOOSELEAF W/CONNECT
6th Edition
ISBN: 9781266060144
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 4, Problem 66P
(a)
Interpretation Introduction
Interpretation: To determine the given
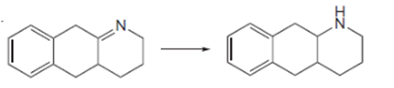
Concept introduction: Oxidation, which refers to the loss of electrons, is the increase in the oxidation state of its component atoms. When an atom obtains electrons or has its oxidation state reduced, the reduction can occur.
(b)
Interpretation Introduction
Interpretation: To determine the given reaction is oxidation and reduction reaction.
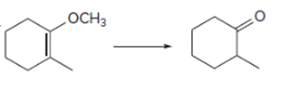
Concept introduction: Oxidation, which refers to the loss of electrons, is the increase in the oxidation state of its component atoms. When an atom obtains electrons or has its oxidation state reduced, the reduction can occur.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
State the products (formulas) of the reaction of acetophenone with iodine and NaOH.
Ch. 4- Precipitation Reactions Worksheet
Write balanced, complete ionic, and net ionic equations for the following reactions that mav
produce precipitates. Use NR to indicate no reaction
Ave
1\
+3
=6
Fe
+
V-2
Na S04
13. Write the balanced equation for the reaction of iron (III) phosphate with sodium sulfate to make
iron (III) sulfate and sodium phosphate.
2FePO4 + M, Soy
a) If you perform this reaction with 25 grams of iron (III) phosphate and an excess of sodium
sulfate, how many grams of iron (III) sulfate can you make?
21 Fe 2
3x 1 Na 3
25g Fe
Ingle
150,829
Indes
2 nol
3
1335
349.89
35.90
Ihol & Sanz
Fez Bak
heck
3x1 50ab) If 18.5 grams of iron (III) sulfate are actually made when you do this reaction, what is your
Poy
percent yield?
118.5
259-1-100
51.4%
(0.74)x100610
335
If you do this reaction with 15 grams of sodium sulfate and get a 65.0% yield, how many
grams of sodium phosphate will you make?
10.59
14. Ammonia is produced from the reaction of nitrogen and hydrogen according…
==
Functional Groups
Identifying and drawing hemiacetals and acetals
In the drawing area below, create an acetal with 1 isopropoxy group, 1 hydroxyl group, and a total of 10 carbon atoms.
Explanation
Click and drag to start drawing a
structure.
Check
G
+
Chapter 4 Solutions
ORGANIC CHEMISTRY - LOOSELEAF W/CONNECT
Ch. 4.1 - Prob. 1PCh. 4.1 - Problem 4.2 Which of the following is not another...Ch. 4.1 - Problem 4.3 Draw the five constitutional isomers...Ch. 4.1 - Prob. 4PCh. 4.1 - Prob. 5PCh. 4.2 - Draw the five constitutional isomers that have...Ch. 4.4 - Problem 4.7 Give the IUPAC name for each...Ch. 4.4 - Give the IUPAC name for each compound. a....Ch. 4.4 - Problem 4.9 Give the structure corresponding to...Ch. 4.4 - Prob. 10P
Ch. 4.5 - Give the IUPAC name for each compound.Ch. 4.5 - Give the structure corresponding to each IUPAC...Ch. 4.8 - Arrange the following compounds in order of...Ch. 4.9 - Problem 4.14 Draw the staggered and eclipsed...Ch. 4.9 - Prob. 15PCh. 4.9 - Prob. 16PCh. 4.10 - Problem 4.17 a. Draw the three staggered and...Ch. 4.10 - Problem 4.18 Rank the following conformations in...Ch. 4.10 - Problem 4.19 Consider rotation around the...Ch. 4.10 - Calculate the destabilization present in each...Ch. 4.12 - Problem 4.21 Classify the ring carbons as up or...Ch. 4.12 - Problem 4.22 Using the cyclohexane with the C’s...Ch. 4.13 - Draw a second chair conformation for each...Ch. 4.13 - Problem 4.24 Draw both conformations for and...Ch. 4.13 - Problem 4.25 Draw the structure for each compound...Ch. 4.13 - Prob. 26PCh. 4.14 - Prob. 31PCh. 4.14 - Prob. 32PCh. 4.15 - Prob. 33PCh. 4 - Name each alkane using the ball-and-stick model,...Ch. 4 -
4.40 Draw the structure corresponding to each...Ch. 4 - 4.42 Give the IUPAC name for each compound.
a....Ch. 4 - Prob. 42PCh. 4 - 4.46 Considering rotation around the bond...Ch. 4 - 4.50 Calculate the barrier to rotation for each...Ch. 4 - 4.51 The eclipsed conformation of is less...Ch. 4 - (a) Draw the anti and gauche conformations for...Ch. 4 - For each compound drawn below: a.Label each OH,Br...Ch. 4 - Draw the two possible chair conformations for...Ch. 4 - For each compound drawn below: a. Draw...Ch. 4 - Classify each pair of compounds as constitutional...Ch. 4 - Prob. 66PCh. 4 - 4.64 Draw the products of combustion of each...Ch. 4 - 4.65 Hydrocarbons like benzene are metabolized in...Ch. 4 - Prob. 69PCh. 4 - Prob. 70PCh. 4 - Cyclopropane and cyclobutane have similar strain...Ch. 4 - Prob. 72PCh. 4 - Haloethanes (CH3CH2X,X=Cl,Br,I) have similar...Ch. 4 - Prob. 74PCh. 4 - Prob. 75PCh. 4 - Consider the tricyclic structure B (a) Label each...Ch. 4 - Read Appendix B on naming branched alkyl...Ch. 4 - Read Appendix B on naming bicyclic compounds. Then...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- State the products (formulas) of the reaction of acetophenone with iodine and NaOH.arrow_forwardExplanation Check Draw the skeletal ("line") structure of 5-hydroxy-4-methyl-2-pentanone. Click and drag to start drawing a structure. Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Cer ☐ : Carrow_forward1. Using a Model set Build a model for the following compound [CH2BrCI]. 2. Build another model of the mirror image of your first molecule. 3. Place the two models next to each other and take a picture which shows the differences between the two models. 4. Determine the absolute stereochemistry R or S for the two models. 5. Write or type a paragraph to Discuss the stereochemical relationship between the two models of CH2BrCl. You must provide an explanation for your conclusions also provide a description for the colors used to represent each atom in the model's images.arrow_forward
- What parameters are included in the specific rotation calculation of a pure substance based on measurement from a polarimeter? Select one or more: Density of the sample Pathlength of the sample container Enantiomeric excess of the sample Measured rotation of lightarrow_forwardV Determine whether the following molecule is a hemiacetal, acetal, or neither and select the appropriate box below. Also, highlight the hemiacetal or acetal carbon if there is one. Explanation O CH O Ohemiacetal Oacetal Oneither Check A 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Cer 000 Ararrow_forward1. Using Online resources and chemical structures hand draw four different organic compounds (not those already shown in your handout) that are chiral, optically active (a pair of enantiomers will count as one). Pay attention to correct stereochemistry 2. Write or type a short paragraph to Discuss the stereochemical relationship between the four compounds.arrow_forward
- 1. Using a Model set Build a model for the following compound [CHBRIF] 2. Build another model of the mirror image of your first molecule. 3. Place the two models next to each other and take a picture which shows the differences between the two models. 4. Determine the absolute stereochemistry R or S for the two models. 5. Write or type a paragraph to Discuss the stereochemical relationship between the two models of CHBгCIF. You must provide an explanation for your conclusions also provide a description for the colors used to representarrow_forwardThe specific rotation of a sample depends upon measured angle of rotation, the density of the sample, and the pathway length of the light. True Falsearrow_forwardConsider the molecule A,B, C and D shown below, (1 x 4) Br NH2 A OH Br 边 H B C D 1. Assign the R/S configuration to each chiral center and identify by circling all the chiral centers. 2. Draw an image for the enantiomer of each of the compounds A, B, C and D.arrow_forward
- Could you crystallize one enantiomer of mandelic acid from a racemic mixture (using the typical achiral solvents found in our lab) without preparing a diastereomeric salt? Why or why not? No, because both enantiomers have the same solubility in achiral solvents. than the other. ооо Yes, because one enantiomer has a higher melting point No, because both enantiomers are liquids. Yes, because one enantiomer is more crystalline than the other.arrow_forwardIf the literature value of specific rotation for a chiral compound is -53.6°, what is the enantiomeric excess of a compound with a measured specific rotation of -40.5°?arrow_forwardThe process to determine the configuration, starts by placing the lowest priority substituent toward the back. If the substituents pointing forward decrease in priority in a clockwise order, the configuration is S. If the substituents decrease in priority in a counterclockwise order, the configuration is R. True Falsearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning