![OWLv2 for Moore/Stanitski's Chemistry: The Molecular Science, 5th Edition, [Instant Access], 1 term (6 months)](https://s3.amazonaws.com/compass-isbn-assets/textbook_empty_images/large_textbook_empty.svg)
Concept explainers
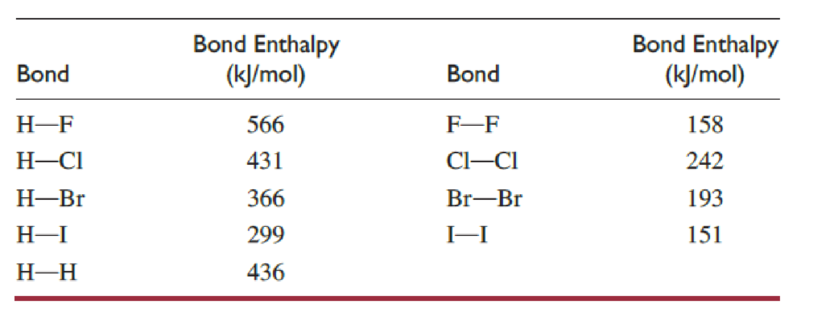
For the reactions of molecular hydrogen with fluorine and with chlorine:
- (a) Calculate the enthalpy change for breaking all the bonds in the reactants.
- (b) Calculate the enthalpy change for forming all the bonds in the products.
- (c) From the results in parts (a) and (b), calculate the enthalpy change for the reaction.
- (d) Which reaction is most exothermic?
(a)
Interpretation:
The enthalpy change for breaking of all bonds present in all reactants of given reaction has to be calculated.
Answer to Problem 61QRT
The enthalpy change value for breaking of bonds for fluorine reaction is
Explanation of Solution
The reaction of molecular hydrogen with fluorine and chlorine is as follows,
Above both reactions, involves breaking of1
(b)
Interpretation:
The enthalpy change for forming of all bonds present in all products of given reaction has to be calculated.
Answer to Problem 61QRT
The enthalpy change value for forming of bonds in fluorine reaction is
Explanation of Solution
The reaction of molecular hydrogen with fluorine and chlorine is as follows,
Above both reactions involve formation of 2 hydrogen-halogen bonds. The
(c)
Interpretation:
The enthalpy change given reaction has to be calculated.
Concept Introduction:
The enthalpy change in a system
Where,
Answer to Problem 61QRT
The enthalpy change value for fluorine reaction is
Explanation of Solution
The reaction of molecular hydrogen with fluorine and chlorine is as follows,
The enthalpy change value for each reaction is determined by considering the formula,
(d)
Interpretation:
From the two given reactions, the exothermic reaction has to be identified.
Concept Introduction:
Enthalpy is the amount energy absorbed or released in a process. Under constant pressure conditions the enthalpy change will be equal to molar q.
Exothermic reaction: Exothermic reactions are those in which evolution of heat takes place during any chemical reaction. They release heat because the reactant molecules require less heat for breakage of bonds than the product molecules.
Endothermic reaction: Endothermic reactions are those in which heat is absorbed during any chemical reaction. In such type of reactions, external energy is needed.
Answer to Problem 61QRT
The reaction between molecular hydrogen and fluorine is more exothermic than the other one.
Explanation of Solution
The reaction of molecular hydrogen with fluorine is more exothermic since
Want to see more full solutions like this?
Chapter 4 Solutions
OWLv2 for Moore/Stanitski's Chemistry: The Molecular Science, 5th Edition, [Instant Access], 1 term (6 months)
- The quantum yield of the photochemical decay of HI is 2. Calculating the moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forwardThe quantum yield of the photochemical decay of HI is 2. Calculate the number of Einsteins absorbed per mole knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forwardThe quantum yield of the photochemical decay of HI is 2. How many moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forward
- If the energy absorbed per mole of photons is 450 kJ, the number of Einsteins absorbed per 1 mole.arrow_forwardWhen propionic aldehyde in vapor form at 200 mmHg and 30°C is irradiated with radiation of wavelength 302 nm, the quantum yield with respect to the formation of CO is 0.54. If the intensity of the incident radiation is 1.5x10-3 W, find the rate of formation of CO.arrow_forwardDraw mechanismarrow_forward
- Does Avogadro's number have units?arrow_forwardExplain why the total E in an Einstein depends on the frequency or wavelength of the light.arrow_forwardIf the dissociation energy of one mole of O2 is 5.17 eV, determine the wavelength that must be used to dissociate it with electromagnetic radiation. Indicate how many Einstein's of this radiation are needed to dissociate 1 liter of O2 at 25°C and 1 atm of pressure.Data: 1 eV = 96485 kJ mol-1; R = 0.082 atm L K-1; c = 2.998x108 m s-1; h = 6.626x10-34 J s; NA = 6.022x 1023 mol-1arrow_forward
- Indicate the number of Einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy (wavelength 475 nm).arrow_forwardIndicate the number of einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy?arrow_forwardA unit used in photochemistry is the einstein. If 400 kJ mol-1 of energy has been absorbed, how many einsteins is this equivalent to?arrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning





