
Consider two chemical changes: one occurring at a tetrahedral
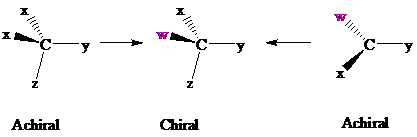
Both transformations convert C in each achiral reactant to a chirality center in the product. The two achiral reactants are classified as prochiral. C is a prochiralitycenter in
In achiral molecules with tetrahedral prochiralitycenters, substitution of one of the two x groups by w gives the enantiomer of the product that results from substitution of the other. The two x groups occupy mirror-image sites and are enantiotopic.
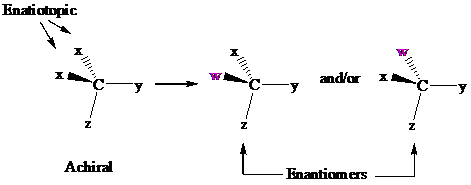
Enantiotopic groups are designated as pro-R or pro-S by a modification of Cahn–Ingold– Prelog notation. One is assigned a higher priority than the other without disturbing the priorities of the remaining groups, and the R,S configuration of the resulting chirality center is determined in the usual way. If it is R, the group assigned the higher rank is pro-R. If S, this group is pro-S. Ethanol and citric acid illustrate the application of this notation to two prochiral molecules.
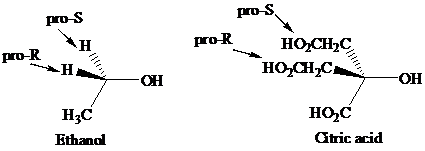
Citric acid played a major role in the development of the concept of prochirality. Its two
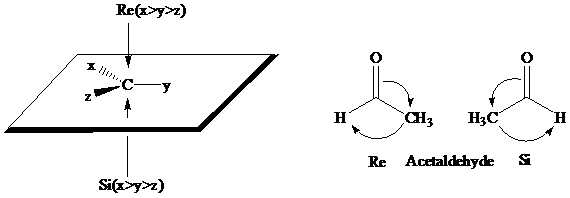
The stereochemical aspects of many enzyme-catalyzed reactions have been determined. Then enzyme alcohol dehydrogenase catalyzes the oxidation of ethanol to acetaldehyde by removing the pro-R hydrogen (abbreviated as HR). When the same enzyme catalyzes the reduction of acetaldehyde to ethanol, hydrogen is transferred to the Re face.
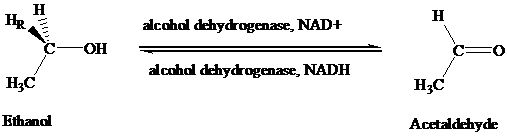
The enzyme fumarasecatalyzes the addition of water to the double bond of fumaric acid.
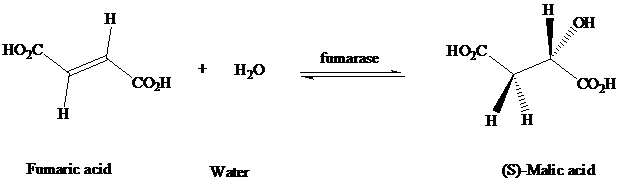
The
A. syn Addition
B. anti Addition
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Organic Chemistry - Standalone book
- Q4: Rank the relative nucleophilicity of halide ions in water solution and DMF solution, respectively. F CI Br | Q5: Determine which of the substrates will and will not react with NaSCH3 in an SN2 reaction to have a reasonable yield of product. NH2 Br Br Br .OH Brarrow_forwardClassify each molecule as optically active or inactive. Determine the configuration at each H соон Chirality center OH 애 He OH H3C Ноос H H COOH A K B.arrow_forwardQ1: Rank the relative nucleophilicity of the following species in ethanol. CH3O¯, CH3OH, CH3COO, CH3COOH, CH3S Q2: Group these solvents into either protic solvents or aprotic solvents. Acetonitrile (CH3CN), H₂O, Acetic acid (CH3COOH), Acetone (CH3COCH3), CH3CH2OH, DMSO (CH3SOCH3), DMF (HCON(CH3)2), CH3OHarrow_forward
- Don't used hand raiting and don't used Ai solutionarrow_forward10. The main product of the following reaction is [1.1:4',1"-terphenyl]-2'-yl(1h-pyrazol-4- yl)methanone Ph N-H Pharrow_forwardDraw the Fischer projection for a D-aldo-pentose. (aldehyde pentose). How many total stereoisomers are there? Name the sugar you drew. Draw the Fischer projection for a L-keto-hexose. (ketone pentose). How many total stereoisomers are there? Draw the enantiomer.arrow_forward
- Draw a structure using wedges and dashes for the following compound: H- Et OH HO- H H- Me OHarrow_forwardWhich of the following molecules are NOT typical carbohydrates? For the molecules that are carbohydrates, label them as an aldose or ketose. HO Он ОН ОН Он ОН но ΤΗ HO ОН HO eve Он он ОН ОН ОН If polyethylene has an average molecular weight of 25,000 g/mol, how many repeat units are present?arrow_forwardDraw the a-anomer cyclized pyranose Haworth projection of the below hexose. Circle the anomeric carbons. Number the carbons on the Fischer and Haworth projections. Assign R and S for each chiral center. HO CHO -H HO -H H- -OH H -OH CH₂OH Draw the ẞ-anomer cyclized furanose Haworth projection for the below hexose. Circle the anomeric carbons. Number the carbons on the Fischer and Haworth projections. HO CHO -H H -OH HO -H H -OH CH₂OHarrow_forward

 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


