
Consider two chemical changes: one occurring at a tetrahedral
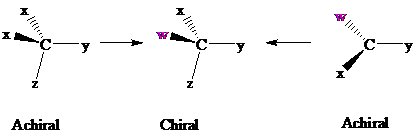
Both transformations convert C in each achiral reactant to a chirality center in the product. The two achiral reactants are classified as prochiral. C is a prochiralitycenter in
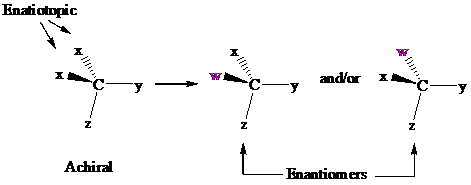
In achiral molecules with tetrahedral prochiralitycenters, substitution of one of the two x groups by w gives the enantiomer of the product that results from substitution of the other. The two x groups occupy mirror-image sites and are enantiotopic.
Enantiotopic groups are designated as pro-R or pro-S by a modification of Cahn–Ingold– Prelog notation. One is assigned a higher priority than the other without disturbing the priorities of the remaining groups, and the R,S configuration of the resulting chirality center is determined in the usual way. If it is R, the group assigned the higher rank is pro-R. If S, this group is pro-S. Ethanol and citric acid illustrate the application of this notation to two prochiral molecules.
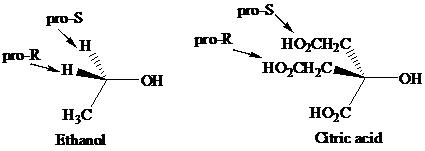
Citric acid played a major role in the development of the concept of prochirality. Its two
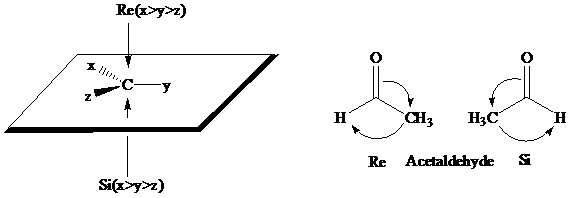
The stereochemical aspects of many enzyme-catalyzed reactions have been determined. Then enzyme alcohol dehydrogenase catalyzes the oxidation of ethanol to acetaldehyde by removing the pro-R hydrogen (abbreviated as HR). When the same enzyme catalyzes the reduction of acetaldehyde to ethanol, hydrogen is transferred to the Re face.
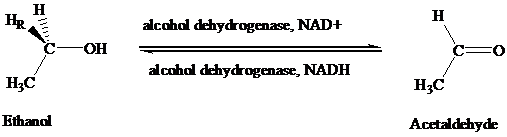
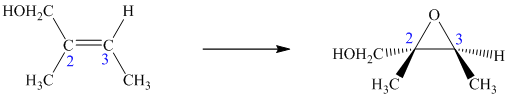
A method for the stereoselective synthesis of chiral
Re Re
Re Si
Si Si
Si Re
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Organic Chemistry - Standalone book
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward

 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


