
Concept explainers
(a)
Interpretation:
The equilibrium constant for interconversion of given
Concept introduction:
The free energy diagram of a reaction is the plot of standard free energy versus reaction coordinate or reaction progress. The products and reactants are placed at their respective free energy. The difference in the free energy of products and reactants is the standard free energy of the reaction.
Answer to Problem 4.68AP
The equilibrium constant for interconversion of given alkenes is
The alkene that is more favorable is shown below.
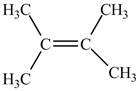
Explanation of Solution
The given alkenes undergoing interconversion along with their free energy of formation are shown below.
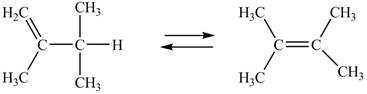
Figure 1
The free energy change for the interconversion of alkenes is equal to the free energy of formation of the product minus the free energy of formation of the reactant.
Substitute the free energy of formation of product alkene and reactant alkene in the equation (1) as shown below.
The Gibbs free energy of the reaction is related to its equilibrium constant by the relation shown below.
Where,
•
•
The value of
Substitute the value of
Rearrange above equation to calculate the
Take the antilog on both sides of the equation as shown below.
The equilibrium constant for the interconversion of alkenes is
The value of equilibrium constant is high, therefore, the alkene on the product is more favored. This can also be understood from the negative value of Gibbs free energy of the reaction which indicates that the reaction is spontaneous. Therefore, alkene on the product side is more favorable which is shown below.
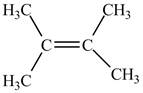
Figure 2
The equilibrium constant for interconversion of given alkenes is
The alkene that is more favorable is shown in Figure 2.
(b)
Interpretation:
The information of the rate at which the interconversion is taking place from the equilibrium constant value is to be stated.
Concept introduction:
The equilibrium constant of the reaction gives information about the
Answer to Problem 4.68AP
The rate of interconversion of alkene is moderate. The rate of forward reaction is
Explanation of Solution
The equilibrium constant in terms of the concentration of reactant alkene and product alkene is shown below.
The value of the equilibrium constant is
Substitute the value of the equilibrium constant in the above expression.
The concentration of product alkene at equilibrium is only
The equilibrium constant of the reaction is the ratio of rate constant of forward reaction and backward reaction.
The rate of interconversion of alkene is moderate from the value of the equilibrium constant.
Want to see more full solutions like this?
Chapter 4 Solutions
EBK ORGANIC CHEMISTRY
- One liter of chlorine gas at 1 atm and 298 K reacts completely with 1.00 L of nitrogen gas and 2.00 L of oxygen gas at the same temperature and pressure. A single gaseous product is formed, which fills a 2.00 L flask at 1.00 atm and 298 K. Use this information to determine the following characteristics of the product:(a) its empirical formula;(b) its molecular formula;(c) the most favorable Lewis formula based on formal charge arguments (the central atom is N);(d) the shape of the molecule.arrow_forwardHow does the square root mean square velocity of gas molecules vary with temperature? Illustrate this relationship by plotting the square root mean square velocity of N2 molecules as a function of temperature from T=100 K to T=300 K.arrow_forwardDraw product B, indicating what type of reaction occurs. F3C CF3 NH2 Me O .N. + B OMearrow_forward
- Benzimidazole E. State its formula. sState the differences in the formula with other benzimidazoles.arrow_forwardDraw product A, indicating what type of reaction occurs. F3C CN CF3 K2CO3, DMSO, H₂O2 Aarrow_forward19) Which metal is most commonly used in galvanization to protect steel structures from oxidation? Lead a. b. Tin C. Nickel d. Zinc 20) The following molecule is an example of a: R₁ R2- -N-R3 a. Secondary amine b. Secondary amide c. Tertiary amine d. Tertiary amidearrow_forward
- pls helparrow_forwardpls helparrow_forward35) Complete the following equation by drawing the line the structure of the products that are formed. Please note that in some cases more than one product is possible. You must draw all possible products to recive full marks! a. ethanol + 2-propanol + H2SO4 → b. OH conc. H2SO4 CH2 H3C CH + K2Cr2O7 C. d. H3C A pressure CH3 + H2 CH Pt catalystarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





