
4-44 Answer true or false.
(a) The mole is a counting unit, just as a dozen is acounting unit.
(b)
(c) Avogadro’s number, to three significant figures, is 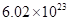 formula units per mole.
formula units per mole.
(d) 1 mol of H2O contains 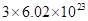 formula units.
formula units.
(e) 1 mol of H2O has the same number of molecules as 1 mol of H2O2.
(f) The molar mass of a compound is its formula weight expressed in amu.
(g) The molar mass of H2O is 18 g/mol.
(h) 1 mol of H2O has the same molar mass as 1 mol of H2O2.
(i) 1 mol of ibuprofen, C13H18O2, contains 33 mol of atoms.
(j) To convert moles to grams, multiply by Avogadro’s number.
(k) To convert grams to moles, divide by molar mass.
(l) 1 mol of H2O contains 1 mol of hydrogen atoms and one mol of oxygen atoms.
(m) 1 mol of H2O contains 2 g of hydrogen atoms and 1 g of oxygen atoms.
(n) 1 mole of H2O contains 18.06 X 10 atoms.
Trending nowThis is a popular solution!

Chapter 4 Solutions
Student Solutions Manual for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th
- How exactly is carbon disulfide used in industry? Specifically, where does it come in during rubber or textile production and what is the chemical processes?arrow_forwardA researcher has developed a new analytical method to determine the percent by mass iron in solids. To test the new method, the researcher purchases a standard reference material sample that is 2.85% iron by mass. Analysis of the iron standard with the new method returns values of 2.75%, 2.89%, 2.77%, 2.81%, and 2.87%. Does the new method produce a result that is significantly different from the standard value at the 95% confidence level?arrow_forwardCreate a drawing of an aceral with at least 2 isopropoxy groups, and a total of 11 carbon atomsarrow_forward
- 4. Predict the major product(s) for each of the following reactions. HBr (1 equiv.) peroxide, A a. b. NBS, peroxide, Aarrow_forwardIn addition to the separation techniques used in this lab (magnetism, evaporation, and filtering), there are other commonly used separation techniques. Some of these techniques are:Distillation – this process is used to separate components that have significantly different boiling points. The solution is heated and the lower boiling point substance is vaporized first. The vapor can be collected and condensed and the component recovered as a pure liquid. If the temperature of the mixture is then raised, the next higher boiling component will come off and be collected. Eventually only non-volatile components will be left in the original solution.Centrifugation – a centrifuge will separate mixtures based on their mass. The mixture is placed in a centrifuge tube which is then spun at a high speed. Heavier components will settle at the bottom of the tube while lighter components will be at the top. This is the technique used to separate red blood cells from blood plasma.Sieving – this is…arrow_forwardBriefly describe a eutectic system.arrow_forward
- man Campus Depa (a) Draw the three products (constitutional isomers) obtained when 2-methyl-3-hexene reacts with water and a trace of H2SO4. Hint: one product forms as the result of a 1,2-hydride shift. (1.5 pts) This is the acid-catalyzed alkene hydration reaction.arrow_forwardNonearrow_forward. • • Use retrosynthesis to design a synthesis Br OHarrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning





