
Concept explainers
(a)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
When the bulkier substituent attached to a cyclohexane ring is in the equatorial position, the chair conformation is more favored. In the disubstituted cyclohexane, the substituents which are on the opposite side of the ring are trans to each other. If there are more than one substituents attached, then the conformation in which maximum substituents are in equatorial position is favored and is more stable. Substituents that are trans to each other in one chair conformation remains trans after the chair flip.
Answer to Problem 4.41P
The most stable conformation of the given molecule is:
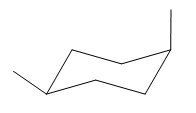
Explanation of Solution
The given compound is:
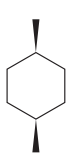
The cyclohexane ring has two methyl groups attached. Both these methyl groups are on the same side of the ring. Thus, they are cis to each other. These two methyl groups are the largest substituents on the ring and each of them is more stable in an equatorial position. Begin by drawing a chair conformation with a methyl group in the equatorial position. It is shown by a wedge bond, hence, it must point up in the chair conformation. It is shown below:
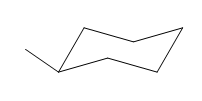
This methyl group is pointed up and the other methyl group must also point up, for them to be cis.

If the chair is flipped, the equatorial methyl group becomes axial and the axial methyl group becomes equatorial. In any case, one methyl group is axial and the other is equatorial.
Hence, this is the most stable chair conformation of the given molecule.
The most stable conformation of the given molecule has one substituent in axial position and other in equatorial position.
(b)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
When the bulkier substituent attached to a cyclohexane ring is in the equatorial position, the chair conformation is more favored. In the disubstituted cyclohexane, the substituents which are on the opposite side of the ring are trans to each other. If there are more than one substituents attached, then the conformation in which maximum substituents are in equatorial position is favored and is more stable. Substituents that are trans to each other in one chair conformation remains trans after the chair flip.
Answer to Problem 4.41P
The most stable conformation of the given molecule is:
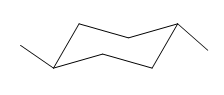
Explanation of Solution
The given compound is:
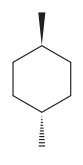
The cyclohexane ring has two methyl groups attached. Both these methyl groups are on the opposite side of the ring. Thus, they are trans to each other. These two methyl groups are the largest substituents on the ring and each of them is more stable in an equatorial position. Begin by drawing a chair conformation with a methyl group in the equatorial position. It is shown by a wedge bond, hence, it must point up in the chair conformation. It is shown below:

This methyl group is pointed up and the other methyl group must point down, for them to be trans.

If the chair is flipped, both the equatorial methyl groups become axial. The chair conformation having both the methyl groups in equatorial position is more stable. Hence, the most stable chair conformation of the given molecule is:

The most stable conformation of the given molecule has both the substituents in the equatorial position.
(c)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
When the bulkier substituent attached to a cyclohexane ring is in the equatorial position, the chair conformation is more favored. In the disubstituted cyclohexane, the substituents which are on the opposite side of the ring are trans to each other. If there are more than one substituents attached, then the conformation in which maximum substituents are in equatorial position is favored and is more stable. Substituents that are trans to each other in one chair conformation remains trans after the chair flip.
Answer to Problem 4.41P
The most stable conformation of the given molecule is:
Explanation of Solution
The given compound is:
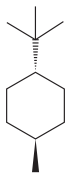
The cyclohexane ring has one methyl group and one tertiary butyl group attached. Both these substituents are on the opposite side of the ring. Thus, they are trans to each other. The tertiary butyl group is the largest substituent on the ring and it is more stable in an equatorial position. Begin by drawing a chair conformation with a tertiary butyl group in the equatorial position. It is shown by a dash bond, hence, it must point down in the chair conformation. It is shown below:
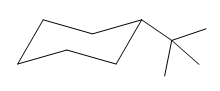
This tertiary butyl group is pointed down and the methyl group must point up, for them to be trans.
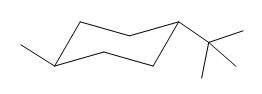
If the chair is flipped, both the equatorial groups become axial. The chair conformation having both the groups in equatorial position is more stable. Hence, the most stable chair conformation of the given molecule is:

The most stable conformation of the given molecule has both the substituents in the equatorial position.
(d)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
When the bulkier substituent attached to a cyclohexane ring is in the equatorial position, the chair conformation is more favored. In the disubstituted cyclohexane, the substituents which are on the opposite side of the ring are trans to each other. If there are more than one substituents attached, then the conformation in which maximum substituents are in equatorial position is favored and is more stable. Substituents that are trans to each other in one chair conformation remains trans after the chair flip.
Answer to Problem 4.41P
The most stable conformation of the given molecule is:
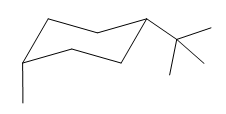
Explanation of Solution
The given compound is:
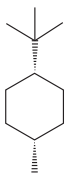
The cyclohexane ring has one methyl group and one tertiary butyl group attached. Both these substituents are on the same side of the ring. Thus, they are cis to each other. The tertiary butyl group is the largest substituent on the ring and it is more stable in an equatorial position. Begin by drawing a chair conformation with a tertiary butyl group in the equatorial position. It is shown by a dash bond, hence, it must point down in the chair conformation. It is shown below:

This tertiary butyl group is pointed down and the methyl group must also point down, for them to be cis.

If the chair is flipped, the equatorial tertiary butyl group becomes axial. The chair conformation having the bulkier tertiary butyl group in equatorial position is more stable. Hence, the most stable chair conformation of the given molecule is:

The most stable conformation of the given molecule has the bulkier substituent in equatorial position and the other substituent in the axial position.
(e)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
When the bulkier substituent attached to a cyclohexane ring is in the equatorial position, the chair conformation is more favored. In the disubstituted cyclohexane, the substituents which are on the opposite side of the ring are trans to each other. If there are more than one substituents attached, then the conformation in which maximum substituents are in equatorial position is favored and is more stable. Substituents that are trans to each other in one chair conformation remains trans after the chair flip.
Answer to Problem 4.41P
The most stable conformation of the given molecule is:
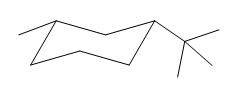
Explanation of Solution
The given compound is:
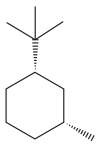
The cyclohexane ring has one methyl group and one tertiary butyl group attached. Both these substituents are on the same side of the ring. Thus, they are cis to each other. The tertiary butyl group is the largest substituent on the ring and it is more stable in an equatorial position. Begin by drawing a chair conformation with a tertiary butyl group in the equatorial position. It is shown by a dash bond, hence, it must point down in the chair conformation. It is shown below:

This tertiary butyl group is pointed down and the methyl group must also point down, for them to be cis.

If the chair is flipped, both the equatorial groups become axial. The chair conformation having both the alkyl groups in equatorial position is more stable. Hence, the most stable chair conformation of the given molecule is:

The most stable conformation of the given molecule has both the substituents in the equatorial position.
(f)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
When the bulkier substituent attached to a cyclohexane ring is in the equatorial position, the chair conformation is more favored. In the disubstituted cyclohexane, the substituents which are on the opposite side of the ring are trans to each other. If there are more than one substituents attached, then the conformation in which maximum substituents are in equatorial position is favored and is more stable. Substituents that are trans to each other in one chair conformation remains trans after the chair flip.
Answer to Problem 4.41P
The most stable conformation of the given molecule is:
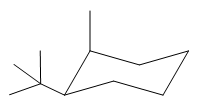
Explanation of Solution
The given compound is:
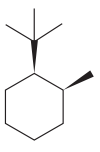
The cyclohexane ring has one tertiary butyl group and one methyl group attached. Both these groups are on the same side of the ring. Thus, they are cis to each other. The tertiary butyl group is the largest substituent on the ring and it is more stable in an equatorial position. Begin by drawing a chair conformation with a tertiary butyl group in the equatorial position. It is shown by a wedge bond, hence, it must point up in the chair conformation. It is shown below:
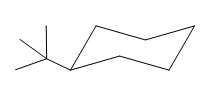
This tertiary butyl group is pointed up and the methyl group must also point up, for them to be cis.

If the chair is flipped, the equatorial tertiary butyl group becomes axial. The chair conformation having the bulkier tertiary butyl group in equatorial position is more stable. Hence, the most stable chair conformation of the given molecule is:

The most stable conformation of the given molecule has the bulkier substituent in equatorial position and the other substituent in axial position.
(g)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
When the bulkier substituent attached to a cyclohexane ring is in the equatorial position, the chair conformation is more favored. In the disubstituted cyclohexane, the substituents which are on the opposite side of the ring are trans to each other. If there are more than one substituents attached, then the conformation in which maximum substituents are in equatorial position is favored and is more stable. Substituents that are trans to each other in one chair conformation remain trans after the chair flip.
Answer to Problem 4.41P
The most stable conformation of the given molecule is:
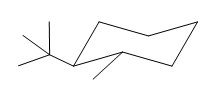
Explanation of Solution
The given compound is:
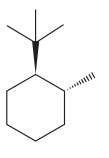
The cyclohexane ring has one tertiary butyl group and one methyl group attached. Both these groups are on the opposite side of the ring. Thus, they are trans to each other. The tertiary butyl group is the largest substituent on the ring and it is more stable in an equatorial position. Begin by drawing a chair conformation with a tertiary butyl group in the equatorial position. It is shown by a wedge bond, hence, it must point up in the chair conformation. It is shown below:

This tertiary butyl group is pointed up and the methyl group must also point down, for them to be trans.

If the chair is flipped, the equatorial tertiary butyl group becomes axial. The chair conformation having the bulkier tertiary butyl group in equatorial position is more stable. Hence, the most stable chair conformation of the given molecule is:

The most stable conformation of the given molecule has both the substituents in the equatorial position.
Want to see more full solutions like this?
Chapter 4 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- Draw the product of the reaction shown below. Use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers, Ignore inorganic byproductsarrow_forwardDraw the product of this reaction please. Ignore inorganic byproductsarrow_forwardOne of the pi molecular orbitals of 1,3-butadiene (CH2=CHCH=CH2) is shown below. Please identify the number of nodal planes perpendicular to the bonding axisarrow_forward
- Draw the monomers required to synthesize this condensation polymer please.arrow_forwardProvide the correct systematic name for the compound shown here. Please take into account the keyboard options belowarrow_forwardcurved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s)arrow_forward
- Identify the 'cartoon' drawing of the acceptor orbital in the first mechanistic step of an electrophilic addition reaction of butadiene with HBr. Pleasearrow_forwardH- H H H H H H Identify and select all structures below that represent a constitutional isomer(s) of the compound shown above. H- H H H A. H H H H-C CI H H D. H H H H H H C C -H H C C H H H H B. H CI H H- C C H H H H E. H CI H C.arrow_forwardWhy doesn't this carry on to form a ring by deprotonating the alpha carbon and the negatively-charged carbon attacking the C=O?arrow_forward
- 6. A solution (0.0004 M) of Fe(S2CNEt2)3 (see the structural drawing below) in chloroform has absorption bands at: 350 nm (absorbance A = 2.34); 514 nm(absorbance A = 0.0532); Calculate the molar absorptivity values for these bands. Comment on their possible nature (charge transfer transitions or d-d S N- transitions?). (4 points)arrow_forwardWhat is the mechanism for this?arrow_forwardFor questions 1-4, consider the following complexes: [Co(CN)6], [COC14]², [Cr(H2O)6]²+ 4. Room temperature (20°C) measurement of molar magnetic susceptibility (Xm) for Fe(NH4)2(SO4)2×6H2O is 1.1888 x 102 cgs (Gaussian units). Calculate effective magnetic moment and provide a number of unpaired electrons for the iron ion. Use this number to rationalize the coordination geometry around iron center. (4 points)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





