
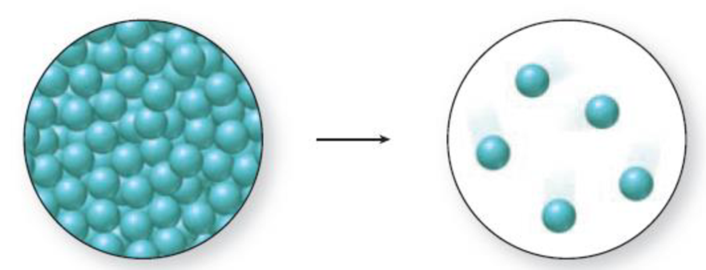
What phase change is shown in the accompanying molecular art? Is energy absorbed or released during the process?
Interpretation:
The phase change accompanying the molecular art and the process of reaction (exothermic and endothermic) has to be given.
The given diagram is,
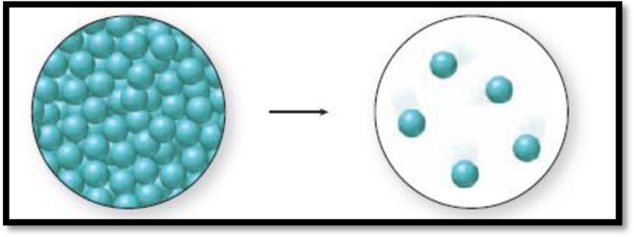
Figure 1
Concept introduction:
Endothermic Reactions:
The endothermic process is a term that describes a reaction where the system absorbs the energy from its surrounding in the form of heat. Few examples of endothermic process is photosynthesis, evaporating liquids, melting ice.
Exothermic reaction:
The exothermic reaction is the opposite of an endothermic reaction. It releases energy by light or heat to its surrounding. Few examples are neutralization, burning a substance, reactions of fuels, deposition of dry ice, respiration.
Vaporization:
Vaporization means conversion of a substance from the liquid phase to the gaseous phase.
Explanation of Solution
The given diagram is,
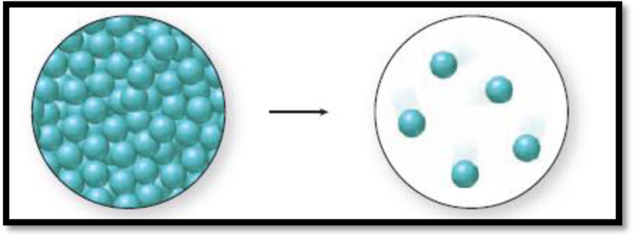
Figure 1
The change in phase can be identified by the distance between the spheres and the level of organization. A solid has closely packed spheres that are well organized, a liquid has closely packed but randomly arranged spheres, and a gas has randomly arranged spheres that are farther away from each other.
The diagram 1 represents a liquid and the diagram two represents a gas. Vaporization is the process of change from liquid to gas. Thus., the phase change accompanied in the figure 1 is a vaporization process and it is an endothermic process because liquid molecules absorb energy to go from liquid state to gaseous state.
Want to see more full solutions like this?
Chapter 4 Solutions
Principles of General, Organic, Biological Chemistry
- here is my question can u help me please!arrow_forwardSo I need help with understanding how to solve these types of problems. I'm very confused on how to do them and what it is exactly, bonds and so forth that I'm drawing. Can you please help me with this and thank you very much!arrow_forwardSo I need help with this problem, can you help me please and thank you!arrow_forward
- Provide steps and explanation please.arrow_forwardDraw a structural formula for the major product of the acid-base reaction shown. H 0 N + HCI (1 mole) CH3 N' (1 mole) CH3 You do not have to consider stereochemistry. ● • Do not include counter-ions, e.g., Na+, I, in your answer. . In those cases in which there are two reactants, draw only the product from 989 CH3 344 ? [Farrow_forwardQuestion 15 What is the major neutral organic product for the following sequence? 1. POCI₂ pyridine ? 2. OsO4 OH 3. NaHSO Major Organic Product ✓ OH OH 'OH OH 'OH 'CIarrow_forward
 World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





