
(a)
Interpretation:
The standard free energy of activation of reaction B is to be calculated.
Concept introduction:
The rate of the reaction is affected by the activation free energy of the reaction. The relationship between the activation free energy and
Where,
•
•
•
Answer to Problem 4.29P
The standard free energy of activation for reaction B is
Explanation of Solution
It is given that standard free energy of activation of reaction A is
The relative rates of two reactions are expressed as,
Where,
•
•
•
Substitute the activation energy for reaction A, the relative rate of A and B, gas constant and temperature in the given formula.
Rearrange the above equation for the calculation of activation energy of B as shown below.
Thus, standard free energy of activation for reaction B is
The standard free energy of activation for reaction B is
(b)
Interpretation:
The reaction free energy diagram for the two reactions showing the two values of
Concept introduction:
The transition state is formed during the conversion of reactants into products in the
Answer to Problem 4.29P
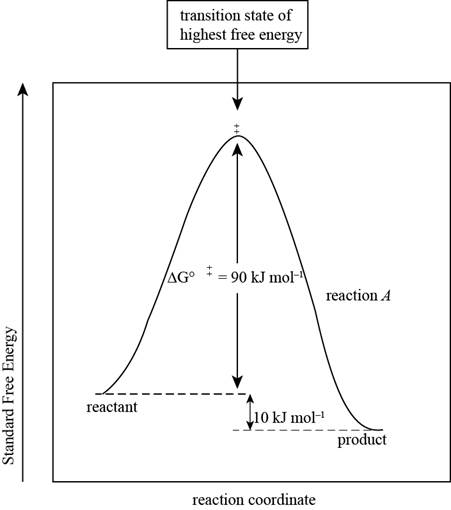
The reaction free-energy diagram for the reaction A showing the two values of
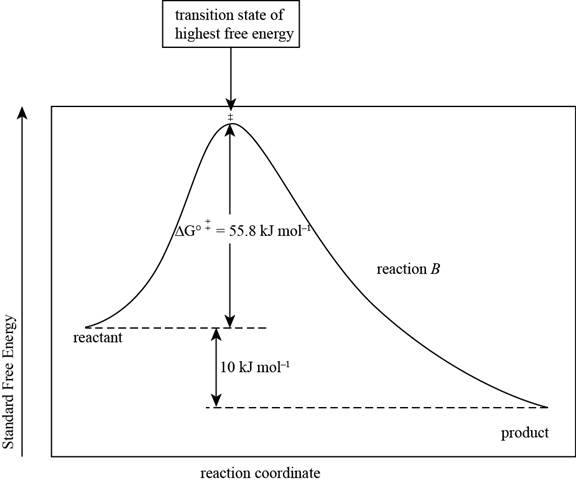
The reaction free-energy diagram for the reaction B showing the two values of
Explanation of Solution
It is given that standard free energy of activation of reaction A is
The reaction free-energy diagram for the reaction A showing the two values of
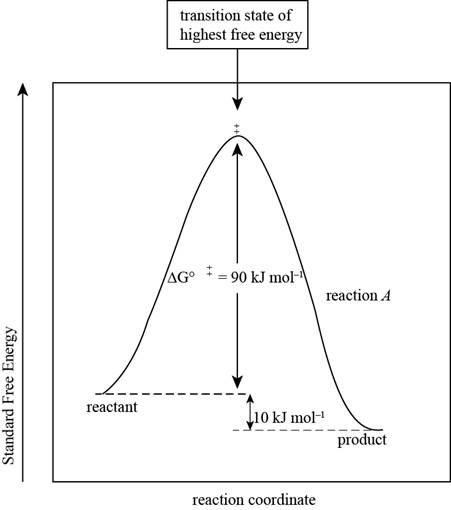
Figure 1
This diagram represents the plot between standard free energy and the reaction coordinates. The point at which the energy is maximum represents the transition state of the reaction.
The reaction free energy diagram for the reaction B showing the two values of
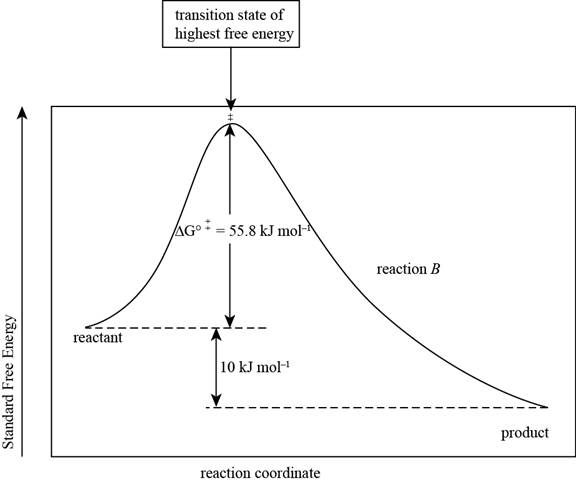
Figure 2
The reaction free-energy diagram for the two reactions showing the two values of
(c)
Interpretation:
The standard free energy of activation of the reverse reaction in both reactions A and B is to be calculated.
Concept introduction:
The free energy diagram represents the plot between standard free energy and the reaction coordinates. The point at which the energy is maximum represents the transition state of the reaction.
Answer to Problem 4.29P
The standard free energy of activation of the reverse reaction in both reactions A and B is
Explanation of Solution
It is given that standard free energy of activation of reaction A is
The products of each reaction are
The standard free energy of activation of the reverse reaction A is,
Thus, the standard free energy of activation of the reverse reaction A is
The standard free energy of activation of the reverse reaction B is,
Thus, the standard free energy of activation of the reverse reaction A is
The standard free energy of activation of the reverse reaction in both reactions A and B is
Want to see more full solutions like this?
Chapter 4 Solutions
EBK ORGANIC CHEMISTRY STUDY GUIDE AND S
- Indicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forward
- Indicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forward
- Indicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are mixed with sodium ethoxide in ethanol.arrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol.arrow_forward2,2-Dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol. Indicate the products obtained.arrow_forward
- Add conditions above and below the arrow that turn the reactant below into the product below in a single transformationADS fint anditions 百 Abl res condinese NC ง Add on condtions 1.0 B H,N.arrow_forward3. Provide all the steps and reagents for this synthesis. OHarrow_forwardSteps and explanationarrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
