
Concept explainers
(a)
Interpretation:
The Newman projection for the species shown is to be drawn looking down the bond indicated in red.
Concept introduction:
A Newman projection is used to visualize the conformation of a molecule by representing it as viewed down the bond of interest. The dot represents front atom, and the circle represents the back atom. The bonds to the front carbon converge on the central point while the bonds to the back carbon end on the circle.
Answer to Problem 4.26P
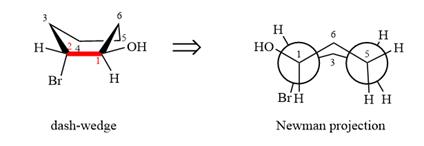
Newman projection of the given structure is:
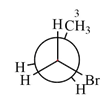
Explanation of Solution
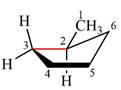
The dash-wedge structure of the molecule is:
The dash-wedge structure shows one hydrogen atom, two carbon atoms on the indicated bond, and the methyl group in one plane. One hydrogen atom on each carbon atom is oriented away from the viewer with bromine, and one hydrogen atom is oriented towards the viewer. For the purpose of determining the positions of the groups in the Newman projection, the carbon bearing the bromine
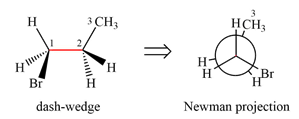
Newman projection represents a molecular structure looking along a particular bond.
(b)
Interpretation:
The Newman projection for the species shown is to be drawn looking down the bond indicated in red.
Concept introduction:
A Newman projection is used to visualize the conformation of a molecule by representing it as viewed down the bond of interest. The dot represents front atom, and the circle represents the back atom. The bonds to the front carbon converge on the central point while the bonds to the back carbon end on the circle.
Answer to Problem 4.26P
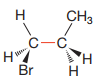
Newman projection of the given structure is:

Explanation of Solution

The dash-wedge structure shows two groups
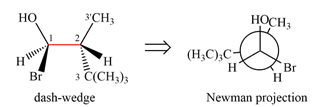
Newman projection represents a molecular structure looking along a particular bond.
(c)
Interpretation:
The Newman projection for the species shown is to be drawn looking down the bond indicated in red.
Concept introduction:
A Newman projection is used to visualize the conformation of a molecule by representing it as viewed down the bond of interest. The dot represents front atom, and the circle represents the back atom. The bonds to the front carbon converge on the central point while the bonds to the back carbon end on the circle.
Answer to Problem 4.26P
Newman projection of the given structure is:
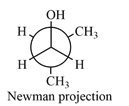
Explanation of Solution
The dash-wedge structure of the molecule is:
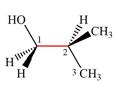
It shows that the carbon on the indicated bond along with
Looking along the indicated bond in
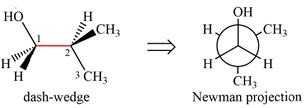
Newman projection represents a molecular structure looking along a particular bond.
(d)
Interpretation:
The Newman projection for the species shown is to be drawn looking down the bond indicated in red.
Concept introduction:
A Newman projection is used to visualize the conformation of a molecule by representing it as viewed down the bond of interest. The dot represents front atom, and the circle represents the back atom. The bonds to the front carbon converge on the central point while the bonds to the back carbon end on the circle.
Answer to Problem 4.26P
Newman projection of the given structure is:
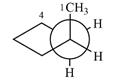
Explanation of Solution
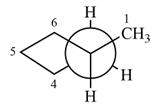
The dash-wedge structure of the molecule is:
The molecule is substituted cyclopentane. The two groups on each of the carbons C2 and C3 are in axial-equatorial positions. On C3, both are hydrogen atoms. On C2, one is a methyl group in the axial position, and the other is hydrogen in equatorial position.
Looking along the indicated bond in
Therefore, the Newman projection of the given molecule can be drawn as:
Newman projection represents a molecular structure looking along a particular bond.
(e)
Interpretation:
The Newman projection for the species shown is to be drawn looking down the bond indicated in red.
Concept introduction:
A Newman projection is used to visualize the conformation of a molecule by representing it as viewed down the bond of interest. The dot represents front atom, and the circle represents the back atom. The bonds to the front carbon converge on the central point while the bonds to the back carbon end on the circle.
Answer to Problem 4.26P
Newman projection of the given structure is:
Explanation of Solution
The dash-wedge structure of the molecule is:
The molecule is substituted cyclopentane. The two groups attached to the ring carbon C2 are methyl in an equatorial up position and hydrogen in an axially down position. The two groups attached to C3 are both hydrogens, one in axial up position and the other in equatorial down position. Looking down the indicated bond in the
The Newman projection of the molecule can therefore be drawn as

Newman projection represents a molecular structure looking along a particular bond.
(f)
Interpretation:
The Newman projection for the species shown is to be drawn looking down the bond indicated in red.
Concept introduction:
A Newman projection is used to visualize the conformation of a molecule by representing it as viewed down the bond of interest. The dot represents front atom, and the circle represents the back atom. The bonds to the front carbon converge on the central point while the bonds to the back carbon end on the circle.
Answer to Problem 4.26P
Newman projection of the given structure is:
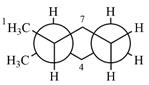
Explanation of Solution
The dash-wedge structure of the molecule is:
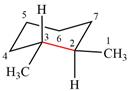
The molecule is substituted cyclohexane in a chair conformation. The methyl groups on the two carbons (C2 and C3) on the indicated bond are both in equatorial positions. The other two groups, both hydrogens, are in axial positions. Looking along the indicated bond in the
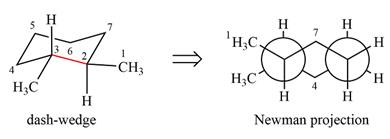
Newman projection represents a molecular structure looking along a particular bond.
(g)
Interpretation:
The Newman projection for the species shown is to be drawn looking down the bond indicated in red.
Concept introduction:
A Newman projection is used to visualize the conformation of a molecule by representing it as viewed down the bond of interest. The dot represents front atom, and the circle represents the back atom. The bonds to the front carbon converge on the central point while the bonds to the back carbon end on the circle.
Answer to Problem 4.26P
Newman projection of the given structure is:
Explanation of Solution
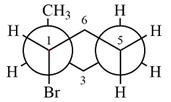
The dash-wedge structure of the molecule is
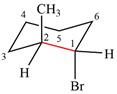
The molecule is substituted cyclohexane in a chair conformation. The two groups attached to C1 are bromine in axial down position and hydrogen in equatorial up position. The two groups attached to C2 are methyl in axial up position and hydrogen in equatorial down position. Looking down the indicated bond, the bromine on the front carbon (C1) and the methyl group on the back carbon (C2), in the Newman projection, will appear straight up and straight down respectively, in staggered positions. The hydrogen on front carbon C1 will appear going to the left, slanted up. The hydrogen on back carbon C2 will appear going left, slanted down. The rest of the ring will appear on right with C6 carbon, on the front. slanted up. C3 carbon, on the back, will appear going to right, slanted down.
The Newman projection of the molecule can then be drawn as
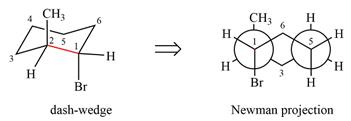
Newman projection represents a molecular structure looking along a particular bond.
(h)
Interpretation:
The Newman projection for the species shown is to be drawn looking down the bond indicated in red.
Concept introduction:
A Newman projection is used to visualize the conformation of a molecule by representing it as viewed down the bond of interest. The dot represents front atom, and the circle represents the back atom. The bonds to the front carbon converge on the central point while the bonds to the back carbon end on the circle.
Answer to Problem 4.26P
Newman projection of the given structure is:
Explanation of Solution
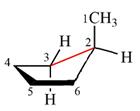
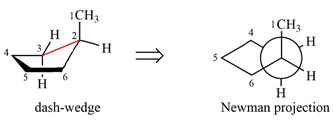
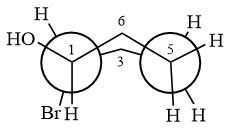
The dash-wedge structure of the molecule is
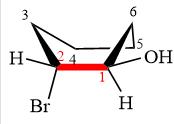
The molecule is substituted cyclohexane in boat conformation. The two groups on C1 are
Looking down the indicated bond, the
Newman projection represents a molecular structure looking along a particular bond.
Want to see more full solutions like this?
Chapter 4 Solutions
Organic Chemistry: Principles And Mechanisms
- Complete the mechanismarrow_forwardV Biological Macromolecules Drawing the Haworth projection of an aldose from its Fischer projection Draw a Haworth projection of a common cyclic form of this monosaccharide: H C=O HO H HO H H OH CH₂OH Explanation Check Click and drag to start drawing a structure. Xarrow_forwardComplete the mechanismarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
