
Concept explainers
Interpretation:
The labeled bonds in the given compound are to be arranged in the order of increasing bond lengths.
Concept introduction:
In hybridization, one
Answer to Problem 4.1P
The increasing order of bond-lengths in the given compound is
Explanation of Solution
The given compound is,
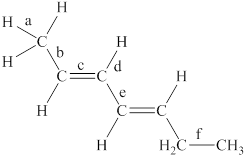
Figure 1
The bond with higher percentage of s-character has electron density closer to the nucleus and thus, has shorter bond length. The order of percentage
• Bond ‘a’ is present between the
• Bond ‘c’ is present between the two
• Due to the conjugation between two pi bonds, the bond length of ‘e’ is in between the carbon-carbon single bond and carbon-carbon double bond.
• Bond ‘b’ is present between one
• The
From the above points, it is concluded that the order of bond-lengths in the given compound is
The increasing order of bond-lengths in the given compound is
Want to see more full solutions like this?
Chapter 4 Solutions
Organic Chemistry, Ebook And Single-course Homework Access
- Show how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid.arrow_forwardHelp me solve this problem. Thank you in advance.arrow_forward22.7 Predict the monoalkylated products of the following reactions with benzene. (a) AlCl3 Ya (b) AlCl3 (c) H3PO4 (d) 22.8 Think-Pair-Share AICI3 The reaction below is a common electrophilic aromatic substitution. SO3 H₂SO4 SO₂H (a) Draw the reaction mechanism for this reaction using HSO,+ as the electrophile. (b) Sketch the reaction coordinate diagram, where the product is lower in energy than the starting reactant. (c) Which step in the reaction mechanism is highest in energy? Explain. (d) Which of the following reaction conditions could be used in an electrophilic aro- matic substitution with benzene to provide substituted phenyl derivatives? (i) AICI3 HNO3 H₂SO4 K2Cr2O7 (iii) H₂SO4 (iv) H₂PO₁arrow_forward
- Is an acid-base reaction the only type of reaction that would cause leavening products to rise?arrow_forwardHelp me understand this! Thank you in advance.arrow_forward22.22 For each compound, indicate which group on the ring is more strongly activating and then draw a structural formula of the major product formed by nitration of the compound. Br CHO (a) CH3 (b) (c) CHO CH3 SO₂H (d) ☑ OCHS NO₂ (e) (f) CO₂H NHCOCH3 NHCOCH, (h) CHS 22.23 The following molecules each contain two aromatic rings. (b) 000-100- H3C (a) (c) Which ring in each undergoes electrophilic aromatic substitution more readily? Draw the major product formed on nitration.arrow_forward
- V Consider this step in a radical reaction: Br: ? What type of step is this? Check all that apply. Draw the products of the step on the right-hand side of the drawing area below. If more than one set of products is possible, draw any set. Also, draw the mechanism arrows on the left-hand side of the drawing area to show how this happens. ⚫ionization termination initialization neutralization none of the abc Explanation Check 80 Ο F3 F1 F2 2 F4 01 % do5 $ 94 #3 X 5 C MacBook Air 25 F5 F6 66 ©2025 ˇ F7 29 & 7 8arrow_forwardShow how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid.arrow_forwardno aiarrow_forward
- Polymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts.arrow_forwardDraw a tetramer if this alternating copolymer pleasearrow_forwardDraw the monomers required to synthesize this condensation polymer.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
