
Concept explainers
(a)
Interpretation:
The systematic name of given compounds has to be provided.
Concept Introduction:
Nomenclature of organic compounds:
The naming of the organic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
- The longer number of Carbon chain of a compound is identified this is called parent of the compound.
- In the cyclic compounds the number of carbon involving in ring formation is called parent of the compound.
- The compound have more than one parent chains means the larger number of substitutions present in the chain is consider as a parent chain.
- The names of all substituents are arranged by alphabets to starts with lowest numbering.
- In the complex substituent having compounds the substituent name is assigned by a name each of them based on numbers going away from the parent.
(a)
Explanation of Solution
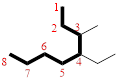
In the given compound, the longest carbon chain (highlighted with bold lines) contains EIGHT carbons and while numbering the parent chain, substituents should get the least possible number.
The parent name is OCTANE. The substituents are arranged in alphabetical order followed by the parent name.
Then as usual, the substituents are arranged in alphabetical order as ‘4-ethyl-3-methyl-’
Therefore, the systematic name of the given compounds is ‘4-ethyl-3-methyloctane’.
(b)
Interpretation:
The systematic name of given compounds has to be provided.
Concept Introduction:
Nomenclature of organic compounds:
The naming of the organic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
- The longer number of Carbon chain of a compound is identified this is called parent of the compound.
- In the cyclic compounds the number of carbon involving in ring formation is called parent of the compound.
- The compound have more than one parent chains means the larger number of substitutions present in the chain is consider as a parent chain.
- The names of all substituents are arranged by alphabets to starts with lowest numbering.
- In the complex substituent having compounds the substituent name is assigned by a name each of them based on numbers going away from the parent.
(b)
Explanation of Solution
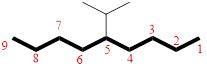
In the given compound, the longest carbon chain (highlighted with bold lines) contains NINE carbons and while numbering the parent chain, substituents should get the least possible number. The parent name is NONANE. The substituents are arranged in alphabetical order followed by the parent name.
Then as usual, the substituents are arranged in alphabetical order as ‘5-isopropyl’
Therefore, the systematic name of the given compounds is ‘5-isopropylnonane’.
(c)
Interpretation:
The systematic name of given compounds has to be provided.
Concept Introduction:
Nomenclature of organic compounds:
The naming of the organic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
- The longer number of Carbon chain of a compound is identified this is called parent of the compound.
- In the cyclic compounds the number of carbon involving in ring formation is called parent of the compound.
- The compound have more than one parent chains means the larger number of substitutions present in the chain is consider as a parent chain.
- The names of all substituents are arranged by alphabets to starts with lowest numbering.
- In the complex substituent having compounds the substituent name is assigned by a name each of them based on numbers going away from the parent.
(c)
Explanation of Solution
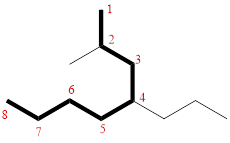
In the given compound, the longest carbon chain (highlighted with bold lines) contains EIGHT carbons and while numbering the parent chain, substituents should get the least possible number.
The parent name is OCTANE. The substituents are arranged in alphabetical order followed by the parent name.
Then as usual, the substituents are arranged in alphabetical order as ‘2-methyl-4-propyl’
Therefore, the systematic name of the given compounds is ‘2-methyl-4-propyloctane’.
(d)
Interpretation:
The systematic name of given compounds has to be provided.
Concept Introduction:
Nomenclature of organic compounds:
The naming of the organic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
- The longer number of Carbon chain of a compound is identified this is called parent of the compound.
- In the cyclic compounds the number of carbon involving in ring formation is called parent of the compound.
- The compound have more than one parent chains means the larger number of substitutions present in the chain is consider as a parent chain.
- The names of all substituents are arranged by alphabets to starts with lowest numbering.
- In the complex substituent having compounds the substituent name is assigned by a name each of them based on numbers going away from the parent.
(d)
Explanation of Solution
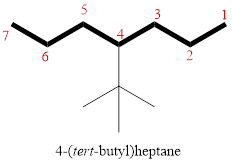
In the given compound, the longest carbon chain (highlighted with bold lines) contains SIX carbons and while numbering the parent chain, substituents should get the least possible number.
The parent name is HEXANE. The substituents are arranged in alphabetical order followed by the parent name.
Then as usual, the substituents are arranged in alphabetical order as ‘4-(tert-butyl)’
Therefore, the systematic name of the given compounds is ‘4-(tert-butyl)heptane’.
(e)
Interpretation:
The systematic name of given compounds has to be provided.
Concept Introduction:
Nomenclature of organic compounds:
The naming of the organic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
- The longer number of Carbon chain of a compound is identified this is called parent of the compound.
- In the cyclic compounds the number of carbon involving in ring formation is called parent of the compound.
- The compound have more than one parent chains means the larger number of substitutions present in the chain is consider as a parent chain.
- The names of all substituents are arranged by alphabets to starts with lowest numbering.
- In the complex substituent having compounds the substituent name is assigned by a name each of them based on numbers going away from the parent.
(e)
Explanation of Solution
In the given compound, the longest carbon chain (highlighted with bold lines) contains ELEVEN carbons and while numbering the parent chain, substituents should get the least possible number.
The parent name is UNDECANE. The substituents are arranged in alphabetical order followed by the parent name.
Then as usual, the substituents are arranged in alphabetical order as ‘5-(sec-butyl)-4-ethyl-2-methyl’
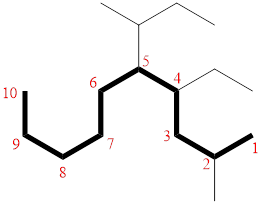
Therefore, the systematic name of the given compounds is ‘5-(sec-butyl)-4-ethyl-2-methyldecane’.
(f)
Interpretation:
The systematic name of given compounds has to be provided.
Concept Introduction:
Nomenclature of organic compounds:
The naming of the organic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
- The longer number of Carbon chain of a compound is identified this is called parent of the compound.
- In the cyclic compounds the number of carbon involving in ring formation is called parent of the compound.
- The compound have more than one parent chains means the larger number of substitutions present in the chain is consider as a parent chain.
- The names of all substituents are arranged by alphabets to starts with lowest numbering.
- In the complex substituent having compounds the substituent name is assigned by a name each of them based on numbers going away from the parent.
(f)
Explanation of Solution
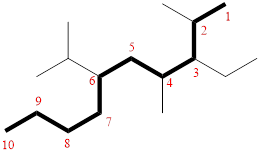
In the given compound, the longest carbon chain (highlighted with bold lines) contains TEN carbons and while numbering the parent chain, substituents should get the least possible number.
The parent name is DECANE. The substituents are arranged in alphabetical order followed by the parent name.
Then as usual, the substituents are arranged in alphabetical order as ‘3-ethyl-6-isopropyl-2,4-dimethyl’
Therefore, the systematic name of the given compounds is ‘3-ethyl-6-isopropyl-2,4-dimethyl decane’.
(g)
Interpretation:
The systematic name of given compounds has to be provided.
Concept Introduction:
Nomenclature of organic compounds:
The naming of the organic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
- The longer number of Carbon chain of a compound is identified this is called parent of the compound.
- In the cyclic compounds the number of carbon involving in ring formation is called parent of the compound.
- The compound have more than one parent chains means the larger number of substitutions present in the chain is consider as a parent chain.
- The names of all substituents are arranged by alphabets to starts with lowest numbering.
- In the complex substituent having compounds the substituent name is assigned by a name each of them based on numbers going away from the parent.
(g)
Explanation of Solution
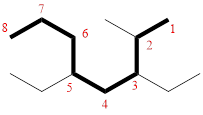
In the given compound, the longest carbon chain (highlighted with bold lines) contains EIGHT carbons and while numbering the parent chain, substituents should get the least possible number.
The parent name is OCTANE. The substituents are arranged in alphabetical order followed by the parent name.
Then as usual, the substituents are arranged in alphabetical order as ‘3,5-diethyl-2-methyl’
Therefore, the systematic name of the given compounds is ‘3,5-diethyl-2-methyloctane’.
(h)
Interpretation:
The systematic name of given compounds has to be provided.
Concept Introduction:
Nomenclature of organic compounds:
The naming of the organic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
- The longer number of Carbon chain of a compound is identified this is called parent of the compound.
- In the cyclic compounds the number of carbon involving in ring formation is called parent of the compound.
- The compound have more than one parent chains means the larger number of substitutions present in the chain is consider as a parent chain.
- The names of all substituents are arranged by alphabets to starts with lowest numbering.
- In the complex substituent having compounds the substituent name is assigned by a name each of them based on numbers going away from the parent.
(h)
Explanation of Solution
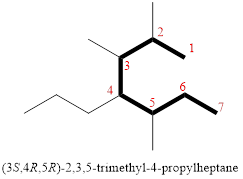
In the given compound, the longest carbon chain (highlighted with bold lines) contains SEVEN carbons and while numbering the parent chain, substituents should get the least possible number.
The parent name is HEPTANE. The substituents are arranged in alphabetical order followed by the parent name.
Then as usual, the substituents are arranged in alphabetical order as ‘2,3,5-trimethyl-4-propyl’
Therefore, the systematic name of the given compounds is ‘2,3,5-trimethyl-4-propylheptane’.
(i)
Interpretation:
The systematic name of given compounds has to be provided.
Concept Introduction:
Nomenclature of organic compounds:
The naming of the organic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
- The longer number of Carbon chain of a compound is identified this is called parent of the compound.
- In the cyclic compounds the number of carbon involving in ring formation is called parent of the compound.
- The compound have more than one parent chains means the larger number of substitutions present in the chain is consider as a parent chain.
- The names of all substituents are arranged by alphabets to starts with lowest numbering.
- In the complex substituent having compounds the substituent name is assigned by a name each of them based on numbers going away from the parent.
(i)
Explanation of Solution
In the given compound, the number of carbon involving in ring formation is called parent ring
(Highlighted with bold lines) contains SIX carbons and while numbering the parent ring, substituents should get the least possible number. The parent name is CYCLOHEXANE. The substituents are arranged in alphabetical order followed by the parent name.
Then as usual, the substituents are arranged in alphabetical order as ‘2,3,5,6-tetramethyl-1-propyl-’
Therefore, the systematic name of the given compounds is ‘2,3,5,6-tetramethyl-1-propyl cyclohexane’.
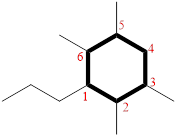
(j)
Interpretation:
The systematic name of given compounds has to be provided.
Concept Introduction:
Bicyclic compounds: Compounds that contain two fused rings called bicyclic compounds.
Nomenclature of bicyclic compounds:
The organic compound naming is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
Identify and the parent: The term ‘Bicylo-’ is introduced in the name of the parent. Count the number of carbons excluding the bridge heads. In the compound below, each of the three paths has two carbons. These three numbers are ordered from largest to smallest, as [2.2.2] and placed in the middle of the parent
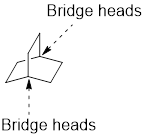
Identify and name substituents: If substituent is present, the parent must be numbered properly in order to assign the locants to the substituent. To number the parent, start at one of the bridgeheads and begin numbering along the longest path, then go to the second longest path, and finally go along the shortest path.
Arrange the substituents alphabetically.
In the complex substituent in compounds, the substituent name is assigned by a name each of them based on numbers going away from the parent.
(j)
Explanation of Solution
The cyclic system has ten carbons and the parent name is DECANE. The term ‘Bicylo-’ is introduced in the name of the parent. On Counting the number of carbons excluding the bridge heads three numbers like (4, 4, and 0) are ordered from largest to smallest, as [4.4.0] and placed in the middle of the parent as bicyclo[4.4.0]decane.
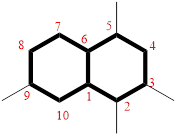
Then as usual, the substituents are arranged in alphabetical order as ‘2,3,5,9-tetramethyl’
Therefore, the systematic name of the given hydrocarbon core is 2,3,5,9-tetramethylbicyclo[4.4.0]decane.
(k)
Interpretation:
The systematic name of given compounds has to be provided.
Concept Introduction:
Bicyclic compounds: Compounds that contain two fused rings called bicyclic compounds.
Nomenclature of bicyclic compounds:
The organic compound naming is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
Identify and the parent: The term ‘Bicylo-’ is introduced in the name of the parent. Count the number of carbons excluding the bridge heads. In the compound below, each of the three paths has two carbons. These three numbers are ordered from largest to smallest, as [2.2.2] and placed in the middle of the parent

Identify and name substituents: If substituent is present, the parent must be numbered properly in order to assign the locants to the substituent. To number the parent, start at one of the bridgeheads and begin numbering along the longest path, then go to the second longest path, and finally go along the shortest path.
Arrange the substituents alphabetically.
In the complex substituent in compounds, the substituent name is assigned by a name each of them based on numbers going away from the parent.
(k)
Explanation of Solution
The cyclic system has ten carbons and the parent name is OCTANE. The term ‘Bicylo-’ is introduced in the name of the parent. On Counting the number of carbons excluding the bridge heads three numbers like (2, 2, and 2) are ordered from largest to smallest, as [2.2.2] and placed in the middle of the parent as bicyclo[2.2.2]octane.
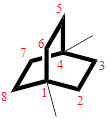
Then as usual, the substituents are arranged in alphabetical order as ‘1,4-dimethyl’
Therefore, the systematic name of the given hydrocarbon core is 1,4-dimethylbicyclo[2.2.2]octane.
Want to see more full solutions like this?
Chapter 4 Solutions
Organic Chemistry, Third Edition Binder Ready Version
- 22.7 Predict the monoalkylated products of the following reactions with benzene. (a) AlCl3 Ya (b) AlCl3 (c) H3PO4 (d) 22.8 Think-Pair-Share AICI3 The reaction below is a common electrophilic aromatic substitution. SO3 H₂SO4 SO₂H (a) Draw the reaction mechanism for this reaction using HSO,+ as the electrophile. (b) Sketch the reaction coordinate diagram, where the product is lower in energy than the starting reactant. (c) Which step in the reaction mechanism is highest in energy? Explain. (d) Which of the following reaction conditions could be used in an electrophilic aro- matic substitution with benzene to provide substituted phenyl derivatives? (i) AICI3 HNO3 H₂SO4 K2Cr2O7 (iii) H₂SO4 (iv) H₂PO₁arrow_forwardIs an acid-base reaction the only type of reaction that would cause leavening products to rise?arrow_forwardHelp me understand this! Thank you in advance.arrow_forward
- 22.22 For each compound, indicate which group on the ring is more strongly activating and then draw a structural formula of the major product formed by nitration of the compound. Br CHO (a) CH3 (b) (c) CHO CH3 SO₂H (d) ☑ OCHS NO₂ (e) (f) CO₂H NHCOCH3 NHCOCH, (h) CHS 22.23 The following molecules each contain two aromatic rings. (b) 000-100- H3C (a) (c) Which ring in each undergoes electrophilic aromatic substitution more readily? Draw the major product formed on nitration.arrow_forwardV Consider this step in a radical reaction: Br: ? What type of step is this? Check all that apply. Draw the products of the step on the right-hand side of the drawing area below. If more than one set of products is possible, draw any set. Also, draw the mechanism arrows on the left-hand side of the drawing area to show how this happens. ⚫ionization termination initialization neutralization none of the abc Explanation Check 80 Ο F3 F1 F2 2 F4 01 % do5 $ 94 #3 X 5 C MacBook Air 25 F5 F6 66 ©2025 ˇ F7 29 & 7 8arrow_forwardShow how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid.arrow_forward
- no aiarrow_forwardPolymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts.arrow_forwardDraw a tetramer if this alternating copolymer pleasearrow_forward
- Draw the monomers required to synthesize this condensation polymer.arrow_forwardDraw the monomers required to synthesize this condensation polymer.arrow_forward8:44 PM Sun Apr 13 Earn Freecash.com O Measurement and Matter =1 Setting up a unit conversion 110 Eddie says... ✰ www-awu.aleks.com A student sets up the following equation to convert a measurement. (The ? stands for a number the student is going to calculate.) Fill in the missing part of this equation. Note: your answer should be in the form of one or more fractions multiplied together. (- 4 J kJ -7.0 × 10 ☐ = ? mmol.°C mol °C x10 μ Explanation Check □·□ torox.io Grey Hill LLC. All Rightsarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





