
Concept explainers
- a. Draw the Lewis structure for ethene (ethylene), H2CCH2, a small hydrocarbon with a
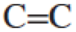 double bond.
double bond. - b. Based on this structure, predict the H–C–H bond angle. Explain your reasoning.
- c. Sketch the molecule showing the predicted bond angles.
(a)
Interpretation:
The Lewis structure for ethylene has to be drawn.
Concept Introduction:
Lewis structures are diagrams that represent the chemical bonding of covalently bonded molecules and coordination compounds.
The chemical bonding present in covalently bonded molecules and in coordination compounds are represented using Lewis structures.
It is also known as Lewis dot structures which represents the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell.
Sometimes the chemical bonding of a molecule cannot be represented using a single Lewis structure. In these cases, the chemical bonding are described by delocalization of electrons and is known as resonance.
All the possible resonance structures are imaginary whereas the resonance hybrid is real.
These structures will differ only in the arrangement of the electrons not in the relative position of the atomic nuclei.
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed such that each atom contains eight electrons in its valence shell.
Molecular geometry is the shape of a molecule predicted by considering only bond pair of electrons.
Geometry of different type of molecules with respect to the number of electron pairs are mentioned below,
Explanation of Solution
The Lewis electron dot structure for given molecules are determined by first drawing the skeletal structure for the given molecules, then the total number of valence electrons for all atoms present in the molecules are determined.
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed considering each atom contains eight electrons in its valence shell.
The given moleucle is ethylene
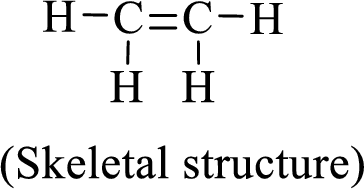
Thus, the Lewis structure of given compound is,
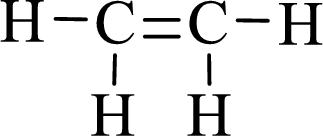
(b)
Interpretation:
The bond angle value in
Concept Introduction:
Molecular geometry is the shape of a molecule predicted by considering only bond pair of electrons.
Geometry of different type of molecules with respect to the number of electron pairs are mentioned below,
Explanation of Solution
Lewis structure for the given compound ethylene is,
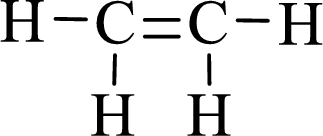
Here, the carbon atom does not have lone pair of electrons and the geometry around the carbon atom is triogonal planar, thus the bond angle in between
(c)
Interpretation:
The ethylene molecule has to be sketched and the predicted bond angle should be shown in the structure.
Concept Introduction:
Molecular geometry is the shape of a molecule predicted by considering only bond pair of electrons.
Geometry of different type of molecules with respect to the number of electron pairs are mentioned below,
Explanation of Solution
Lewis structure for the given compound ethylene is,
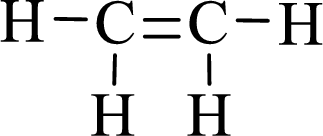
Here, the carbon atom does not have lone pair of electrons and the geometry around the carbon atom is triogonal planar, thus the bond angle in between
According to the predicted bond angle the structure can be sketched as follows,
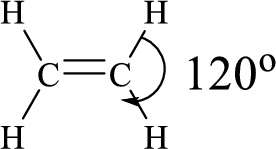
Want to see more full solutions like this?
Chapter 4 Solutions
Chemistry In Context
Additional Science Textbook Solutions
Human Anatomy & Physiology (2nd Edition)
Brock Biology of Microorganisms (15th Edition)
Human Biology: Concepts and Current Issues (8th Edition)
Organic Chemistry (8th Edition)
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning



