
Physics for Scientists and Engineers with Modern Physics
4th Edition
ISBN: 9780131495081
Author: Douglas C. Giancoli
Publisher: Addison-Wesley
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 34, Problem 52GP
A highly reflective mirror can be made for a particular wavelength at normal incidence by using two thin layers of transparent materials of indices of refraction n1 and n2 (1 < n1 < n2) on the surface of the glass (n > n2). What should be the minimum thicknesses d1 and d2 in Fig. 34-29 in terms of the incident wavelength λ, to maximize reflection?
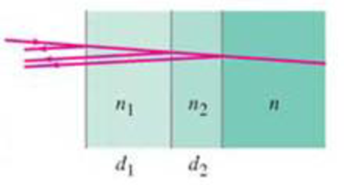
FIGURE 34-29
Problem 52.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Learning Goal:
To understand the meaning and the basic applications of
pV diagrams for an ideal gas.
As you know, the parameters of an ideal gas are
described by the equation
pV = nRT,
where p is the pressure of the gas, V is the volume of
the gas, n is the number of moles, R is the universal gas
constant, and T is the absolute temperature of the gas. It
follows that, for a portion of an ideal gas,
pV
= constant.
Τ
One can see that, if the amount of gas remains constant,
it is impossible to change just one parameter of the gas:
At least one more parameter would also change. For
instance, if the pressure of the gas is changed, we can
be sure that either the volume or the temperature of the
gas (or, maybe, both!) would also change.
To explore these changes, it is often convenient to draw a
graph showing one parameter as a function of the other.
Although there are many choices of axes, the most
common one is a plot of pressure as a function of
volume: a pV diagram.
In this problem, you…
Learning Goal:
To understand the meaning and the basic applications of
pV diagrams for an ideal gas.
As you know, the parameters of an ideal gas are
described by the equation
pV = nRT,
where p is the pressure of the gas, V is the volume of
the gas, n is the number of moles, R is the universal gas
constant, and T is the absolute temperature of the gas. It
follows that, for a portion of an ideal gas,
pV
= constant.
T
One can see that, if the amount of gas remains constant,
it is impossible to change just one parameter of the gas:
At least one more parameter would also change. For
instance, if the pressure of the gas is changed, we can
be sure that either the volume or the temperature of the
gas (or, maybe, both!) would also change.
To explore these changes, it is often convenient to draw a
graph showing one parameter as a function of the other.
Although there are many choices of axes, the most
common one is a plot of pressure as a function of
volume: a pV diagram.
In this problem, you…
■ Review | Constants
A cylinder with a movable piston contains 3.75 mol
of N2 gas (assumed to behave like an ideal gas).
Part A
The N2 is heated at constant volume until 1553 J of heat have been added. Calculate the change in
temperature.
ΜΕ ΑΣΦ
AT =
Submit
Request Answer
Part B
?
K
Suppose the same amount of heat is added to the N2, but this time the gas is allowed to expand while
remaining at constant pressure. Calculate the temperature change.
AT =
Π ΑΣΦ
Submit
Request Answer
Provide Feedback
?
K
Next
Chapter 34 Solutions
Physics for Scientists and Engineers with Modern Physics
Ch. 34.2 - A light beam in air with wavelength = 500 nm,...Ch. 34.4 - What are the values for the intensity I when (a) y...Ch. 34 - Prob. 1QCh. 34 - What is the evidence that light is energy?Ch. 34 - Why is light sometimes described as rays and...Ch. 34 - We can hear sounds around corners but we cannot...Ch. 34 - Can the wavelength of light be determined from...Ch. 34 - Two rays of light from the same source...Ch. 34 - Monochromatic red light is incident on a double...Ch. 34 - If Youngs double-slit experiment were submerged in...
Ch. 34 - Compare a double-slit experiment for sound waves...Ch. 34 - Suppose white light falls on the two slits of Fig....Ch. 34 - Why doesnt the light from the two headlights of a...Ch. 34 - Why are interference fringes noticeable only for a...Ch. 34 - Prob. 13QCh. 34 - Some coated lenses appear greenish yellow when...Ch. 34 - A drop of oil on a pond appears bright at its...Ch. 34 - (II) Derive the law of reflectionnamely, that the...Ch. 34 - (I) Monochromatic light falling on two slits 0.018...Ch. 34 - (I) The third-order bright fringe of 610 nm light...Ch. 34 - (II) Monochromatic light falls on two very narrow...Ch. 34 - (II) If 720-nm and 660-nm light passes through two...Ch. 34 - (II) A red laser from the physics lab is marked as...Ch. 34 - (II) Light of wavelength passes through a pair of...Ch. 34 - (II) Light of wavelength 680 nm falls on two slits...Ch. 34 - (II) A parallel beam of light from a HeNe laser,...Ch. 34 - (II) A physics professor wants to perform a...Ch. 34 - (II) Suppose a thin piece of glass is placed in...Ch. 34 - (II) In a double-slit experiment it is found that...Ch. 34 - (II) Two narrow slits separated by 1.0 mm are...Ch. 34 - (II) In a double-slit experiment, the third-order...Ch. 34 - (II) Light of wavelength 470 nm in air falls on...Ch. 34 - (II) A very thin sheet of plastic (n = 1.60)...Ch. 34 - (I) If one slit in Fig. 3412 is covered, by what...Ch. 34 - (II) Derive an expression similar to Eq. 342 which...Ch. 34 - (II) Show that the angular full width at half...Ch. 34 - (II) In a two-slit interference experiment, the...Ch. 34 - (III) Suppose that one slit of a double-slit...Ch. 34 - (III) (a) Consider three equally spaced and...Ch. 34 - (I) If a soap bubble is 120 nm thick, what...Ch. 34 - (I) How far apart are the dark fringes in Example...Ch. 34 - (II) (a) What is the smallest thickness of a soap...Ch. 34 - (II) A lens appears greenish yellow ( = 570 nm is...Ch. 34 - (II) A thin film of oil (nO = 1.50) with varying...Ch. 34 - (II) A thin oil slick (no = 1.50) finals on water...Ch. 34 - (II) A total of 31 bright and 31 dark Newtons...Ch. 34 - (II) A line metal foil separates one end of two...Ch. 34 - (II) How thick (minimum) should the air layer be...Ch. 34 - (II) A uniform thin film of alcohol (n = 1.36)...Ch. 34 - (II) Show that the radius r of the mth dark...Ch. 34 - (II) Use the result of Problem 33 to show that the...Ch. 34 - (II) When a Newtons ring apparatus (Fig. 3418) is...Ch. 34 - (II) A planoconvex lucite lens 3.4 cm in diameter...Ch. 34 - (II) Lets explore why only thin layers exhibit...Ch. 34 - (II) How far must the mirror M1 in a Michelson...Ch. 34 - (II) What is the wavelength of the light entering...Ch. 34 - (II) A micrometer is connected to the movable...Ch. 34 - (III) One of the beams of an interferometer (Fig,...Ch. 34 - (III) The yellow sodium D lines have wavelengths...Ch. 34 - Prob. 44PCh. 34 - (II) The luminous efficiency of a lightbulb is the...Ch. 34 - Light of wavelength 5.0 107 m passes through two...Ch. 34 - Television and radio waves reflecting from...Ch. 34 - A radio station operating at 88.5 MHz broadcasts...Ch. 34 - Light of wavelength 690 nm passes through two...Ch. 34 - Monochromatic light of variable wavelength is...Ch. 34 - Suppose the mirrors in a Michelson interferometer...Ch. 34 - A highly reflective mirror can be made for a...Ch. 34 - Calculate the minimum thickness needed for an...Ch. 34 - Stealth aircraft are designed to not reflect...Ch. 34 - Light or wavelength strikes a screen containing...Ch. 34 - Consider two antennas radiating 6.0-MHz radio...Ch. 34 - What is the minimum (non-zero) thickness for the...Ch. 34 - Lloyds mirror provides one way of obtaining a...Ch. 34 - Consider the antenna army of Example 345, Fig....Ch. 34 - A thin film of soap (n = 1.34) coats a piece of...Ch. 34 - Two identical sources S1 and S2, separated by...Ch. 34 - A two-slit interference set-up with slit...Ch. 34 - A radio telescope, whose two antennas are...Ch. 34 - In a compact disc (CD), digital information is...
Additional Science Textbook Solutions
Find more solutions based on key concepts
4. Three groups of nonvascular plants are _______, ______, and _______. Three groups of seedless vascular plant...
Biology: Life on Earth (11th Edition)
Modified True/False 1. _____ Biofilms of microorganisms form in aquatic environments only.
Microbiology with Diseases by Body System (5th Edition)
Explain all answers clearly, with complete sentences and proper essay structure if needed. An asterisk(*) desig...
Cosmic Perspective Fundamentals
Some organizations are starting to envision a sustainable societyone in which each generation inherits sufficie...
Campbell Essential Biology (7th Edition)
Differentiate between these terms: chromosome, chromatin, and chromatid.
Campbell Biology (11th Edition)
If someone at the other end of a room smokes a cigarette, you may breathe in some smoke. The movement of smoke ...
Campbell Essential Biology with Physiology (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 4. I've assembled the following assortment of point charges (-4 μC, +6 μC, and +3 μC) into a rectangle, bringing them together from an initial situation where they were all an infinite distance away from each other. Find the electric potential at point "A" (marked by the X) and tell me how much work it would require to bring a +10.0 μC charge to point A if it started an infinite distance away (assume that the other three charges remains fixed). 300 mm -4 UC "A" 0.400 mm +6 UC +3 UC 5. It's Friday night, and you've got big party plans. What will you do? Why, make a capacitor, of course! You use aluminum foil as the plates, and since a standard roll of aluminum foil is 30.5 cm wide you make the plates of your capacitor each 30.5 cm by 30.5 cm. You separate the plates with regular paper, which has a thickness of 0.125 mm and a dielectric constant of 3.7. What is the capacitance of your capacitor? If you connect it to a 12 V battery, how much charge is stored on either plate? =arrow_forwardLearning Goal: To understand the meaning and the basic applications of pV diagrams for an ideal gas. As you know, the parameters of an ideal gas are described by the equation pV = nRT, where p is the pressure of the gas, V is the volume of the gas, n is the number of moles, R is the universal gas constant, and T is the absolute temperature of the gas. It follows that, for a portion of an ideal gas, PV T = constant. One can see that, if the amount of gas remains constant, it is impossible to change just one parameter of the gas: At least one more parameter would also change. For instance, if the pressure of the gas is changed, we can be sure that either the volume or the temperature of the gas (or, maybe, both!) would also change. To explore these changes, it is often convenient to draw a graph showing one parameter as a function of the other. Although there are many choices of axes, the most common one is a plot of pressure as a function of volume: a pV diagram. In this problem, you…arrow_forwardA-e pleasearrow_forward
- Two moles of carbon monoxide (CO) start at a pressure of 1.4 atm and a volume of 35 liters. The gas is then compressed adiabatically to 1/3 this volume. Assume that the gas may be treated as ideal. Part A What is the change in the internal energy of the gas? Express your answer using two significant figures. ΕΠΙ ΑΣΦ AU = Submit Request Answer Part B Does the internal energy increase or decrease? internal energy increases internal energy decreases Submit Request Answer Part C ? J Does the temperature of the gas increase or decrease during this process? temperature of the gas increases temperature of the gas decreases Submit Request Answerarrow_forwardYour answer is partially correct. Two small objects, A and B, are fixed in place and separated by 2.98 cm in a vacuum. Object A has a charge of +0.776 μC, and object B has a charge of -0.776 μC. How many electrons must be removed from A and put onto B to make the electrostatic force that acts on each object an attractive force whose magnitude is 12.4 N? e (mea is the es a co le E o ussian Number Tevtheel ed Media ! Units No units → answe Tr2Earrow_forward4 Problem 4) A particle is being pushed up a smooth slot by a rod. At the instant when 0 = rad, the angular speed of the arm is ė = 1 rad/sec, and the angular acceleration is = 2 rad/sec². What is the net force acting on the 1 kg particle at this instant? Express your answer as a vector in cylindrical coordinates. Hint: You can express the radial coordinate as a function of the angle by observing a right triangle. (20 pts) Ꮎ 2 m Figure 3: Particle pushed by rod along vertical path.arrow_forward
- 4 Problem 4) A particle is being pushed up a smooth slot by a rod. At the instant when 0 = rad, the angular speed of the arm is ė = 1 rad/sec, and the angular acceleration is = 2 rad/sec². What is the net force acting on the 1 kg particle at this instant? Express your answer as a vector in cylindrical coordinates. Hint: You can express the radial coordinate as a function of the angle by observing a right triangle. (20 pts) Ꮎ 2 m Figure 3: Particle pushed by rod along vertical path.arrow_forwardplease solve and answer the question correctly. Thank you!!arrow_forwardNo chatgpt pls will upvotearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax
University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Modern Physics
Physics
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

University Physics Volume 3
Physics
ISBN:9781938168185
Author:William Moebs, Jeff Sanny
Publisher:OpenStax
Polarization of Light: circularly polarized, linearly polarized, unpolarized light.; Author: Physics Videos by Eugene Khutoryansky;https://www.youtube.com/watch?v=8YkfEft4p-w;License: Standard YouTube License, CC-BY