
ORGANIC CHEMISTRY W/BIOLOGICAL TOPICS
6th Edition
ISBN: 9781260325294
Author: SMITH
Publisher: RENT MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3.2, Problem 3P
Classify a carbon atom by the number of carbons to which it is bonded can also be
done in more complex molecules that contain heteroatoms. Classify each
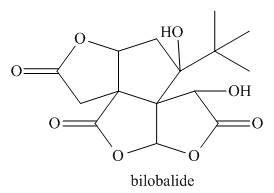
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(EXM 2, PRBLM 3) Here is this problem, can you explain it to me and show how its done. Thank you I need to see the work for like prbl solving.
can someone draw out the reaction mechanism for this reaction showing all bonds, intermediates and side products
Comment on the general features of the 1H-NMR spectrum of isoamyl ester provided below
What would be the best choices for the missing reagents 1 and 3 in this synthesis?
1. PPh3
3
2. n-BuLi
• Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like.
• Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is.
• Note: if one of your reagents needs to contain a halogen, use bromine.
Click and drag to start drawing a structure.
Chapter 3 Solutions
ORGANIC CHEMISTRY W/BIOLOGICAL TOPICS
Ch. 3.1 - Prob. 1PCh. 3.2 - (a) Classify the carbon atoms in each compound as...Ch. 3.2 - Problem 3.3 Classify a carbon atom by the number...Ch. 3.2 - Classify each alkyl halide and alcohol as , or...Ch. 3.2 - Prob. 5PCh. 3.2 - Prob. 6PCh. 3.2 - Draw the structure of a compound of molecular...Ch. 3.2 - Prob. 8PCh. 3.2 - Prob. 9PCh. 3.2 - Draw the structure of a compound fitting each...
Ch. 3.4 - Predict which compound in each pair has the higher...Ch. 3.4 - Prob. 17PCh. 3.4 - a Label the hydrophobic and hydrophilic portions...Ch. 3.5 - Prob. 21PCh. 3 - 3.29
Identify the functional groups in the...Ch. 3 - Prob. 32PCh. 3 - 3.31 For each alkane: (a) classify each carbon...Ch. 3 - 3.32 Identify the functional groups in each...Ch. 3 - 3.33 Identify each functional group located in the...Ch. 3 - 3.34 (a)Identify the functional groups in...Ch. 3 - Draw seven constitutional isomers with molecular...Ch. 3 - Prob. 38PCh. 3 - Prob. 39PCh. 3 - Prob. 40PCh. 3 - Intramolecular force of attraction are often...Ch. 3 - 3.40 (a) Draw four compounds with molecular...Ch. 3 - 3.41 Rank the compounds in each group in order of...Ch. 3 - Explain why CH3CH2NHCH3 has higher boiling point...Ch. 3 - Prob. 45PCh. 3 - 3.44 Rank the following compounds in order of...Ch. 3 - Prob. 47PCh. 3 - 3.50 Predict the solubility of each of the...Ch. 3 - Prob. 52PCh. 3 - Prob. 53PCh. 3 - 3.53 THC is the active component in marijuana, and...Ch. 3 - Prob. 55PCh. 3 - Prob. 56PCh. 3 - 3.60 Quinapril (trade name Accupril) is a drug...Ch. 3 - 3.61 Answer each question about oxycodone, a...Ch. 3 - Prob. 65PCh. 3 - Prob. 66PCh. 3 - 3.64 Explain why A is less water soluble than B,...Ch. 3 - 3.65 Recall from section 1.10B that there is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify the missing organic reactants in the following reaction: X + Y H+ two steps Note: This chemical equation only focuses on the important organic molecules in the reaction. Additional inorganic or small-molecule reactants or products (like H2O) are not shown. In the drawing area below, draw the skeletal ("line") structures of the missing organic reactants X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. Х :arrow_forwardDraw the mechanism of friedel-crafts acylation using acetyl chloride of m-Xylenearrow_forwardI need help naming these in IUPACarrow_forward
- H R Part: 1/2 :CI: is a/an electrophile Part 2 of 2 Draw the skeletal structure of the product(s) for the Lewis acid-base reaction. Include lone pairs and formal charges (if applicable) on the structures. 4-7: H ö- H Skip Part Check X :C1: $ % L Fi Click and drag to start drawing a structure. MacBook Pro & ㅁ x G 0: P Add or increase positive formal cha Save For Later Submit ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centearrow_forwardDraw the friedel-crafts acylation mechanism of m-Xylenearrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- 1. Base on this experimental results, how do you know that the product which you are turning in is methyl 3-nitrobenzoate(meta substituted product ) rather than either of the other two products? 2. What observation suggests that at least a small amount of one or both of the other two isomers are in the mother liquor?arrow_forwardExplain Huckel's rule.arrow_forwardhere is my question can u help me please!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
ENVIRONMENTAL POLLUTION; Author: 7activestudio;https://www.youtube.com/watch?v=oxtMFmDTv3Q;License: Standard YouTube License, CC-BY