
Interpretation:
Predict the result of the catalytic hydrogention of natural rubber and will the product obtained show syndiotactic, atactic or isotactic behaviour.
Concept introduction:
Isotactic, syndiotactic, and atactic are the stereochemical forms. The
Syndiotactic are the macromolecules in which the (-R) groups are arranged in an alternate manner along the long chain of the polymer. Gutta-percha is also an example for Syndiotactic polymer.
In atactic form, the substituents are placed in a random manner along the long chain.
The important point to note here is that the polymer obtained from the chain-growth
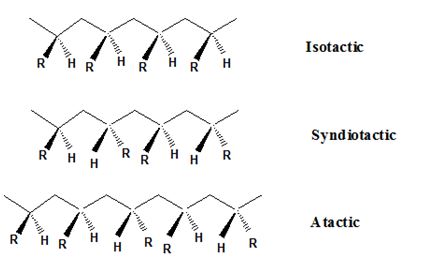
The catalytic hydrogenation natural rubber requires the breaking of double bond and the addition of hydrogen. The double bond of
Therefore, it breaks and hydrogen adds to it. When the hydrogen adds, then the double bonds are replaced with the single bonds. The hydrogenation of double bond releases energy therefore, known as an exothermic reaction. The heat released is called the heat of hydrogenation.
The catalyst used for the purpose of hydrogenation can be Ra-Ni (Raney-Nickel), PtO2 (Platinum oxide), Pd-C (Palladium on carbon) etc. They can be used to enhance the
Trending nowThis is a popular solution!

Chapter 31 Solutions
Bundle: Organic Chemistry, 9th, Loose-Leaf + OWLv2, 4 terms (24 months) Printed Access Card
- A. B. b. Now consider the two bicyclic molecules A. and B. Note that A. is a dianion and B. is a neutral molecule. One of these molecules is a highly reactive compound first characterized in frozen noble gas matrices, that self-reacts rapidly at temperatures above liquid nitrogen temperature. The other compound was isolated at room temperature in the early 1960s, and is a stable ligand used in organometallic chemistry. Which molecule is the more stable molecule, and why?arrow_forwardWhere are the chiral centers in this molecule? Also is this compound meso yes or no?arrow_forwardPLEASE HELP! URGENT!arrow_forward
- Where are the chiral centers in this molecule? Also is this compound meso yes or no?arrow_forwardA mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forward
- A mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forwardHow many chiral carbons are in the molecule? Farrow_forwardcan someone give the curly arrow mechanism for this reaction written with every intermediate and all the side products pleasearrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning


