![OWLv2 with Student Solutions Manual eBook for Masterton/Hurley's Chemistry: Principles and Reactions, 8th Edition, [Instant Access], 4 terms (24 months)](https://www.bartleby.com/isbn_cover_images/9781305863170/9781305863170_largeCoverImage.jpg)
Concept explainers
Magnesium ribbon reacts with acid to produce hydro- gen gas and magnesium ions. Different masses of magnesium ribbon are added to 10 mL of the acid. The volume of the hydrogen gas obtained is a measure of the number of moles of hydrogen produced by the reaction. Various measurements are given in the table below.
(a) Draw a graph of the results by plotting the mass of Mg versus the volume of the hydrogen gas.
(b) What is the limiting reactant in experiment 1?
(c) What is the limiting reactant in experiment 3?
(d) What is the limiting reactant in experiment 6?
(e) Which experiment uses stoichiometric amounts of each reactant?
(f) What volume of gas would be obtained if 0.300 g of Mg ribbon were used? If 0.010 g were used?
(a)
Interpretation:.
The graph between mass of Mg versus the volume of hydrogen gas should be plotted..
Concept introduction:.
The number of moles of a substance is related to mass and molar mass as follows:.
Here,mis mass andMis molar mass of the substance..
The density of solution can be calculated as follow:.
Here, m is mass and V is volume.
Answer to Problem 74QAP
![OWLv2 with Student Solutions Manual eBook for Masterton/Hurley's Chemistry: Principles and Reactions, 8th Edition, [Instant Access], 4 terms (24 months), Chapter 3, Problem 74QAP , additional homework tip 1](https://content.bartleby.com/tbms-images/9781305079373/Chapter-3/images/html_79373-3-74qap_1.png)
Explanation of Solution
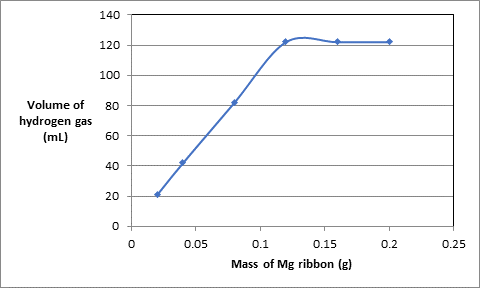
The data of mass of Mg ribbon in grams and volume of hydrogen gas produced in experiments is as follows:.
| Experiment | Mass of Mg ribbon (g) | Volume of acid used (mL) | Volume of hydrogen gas (mL) |
| 1 | 0.020 | 10.0 | 21 |
| 2 | 0.040 | 10.0 | 42 |
| 3 | 0.080 | 10.0 | 82 |
| 4 | 0.120 | 10.0 | 122 |
| 5 | 0.160 | 10.0 | 122 |
| 6 | 0.200 | 10.0 | 122 |
To plot put the data of mass of Mg ribbon on x-axis and volume of hydrogen gas at y-axis:.
![OWLv2 with Student Solutions Manual eBook for Masterton/Hurley's Chemistry: Principles and Reactions, 8th Edition, [Instant Access], 4 terms (24 months), Chapter 3, Problem 74QAP , additional homework tip 2](https://content.bartleby.com/tbms-images/9781305079373/Chapter-3/images/html_79373-3-74qap_2.png)
(b)
Interpretation:
The limiting reactant in experiment 1 should be determined..
Concept introduction:.
The number of moles of a substance is related to mass and molar mass as follows:.
Here,mis mass andMis molar mass of the substance..
The density of solution can be calculated as follow:.
Here, m is mass and V is volume.
Answer to Problem 74QAP
Mg is limiting reactant.
Explanation of Solution
The balanced chemical reaction will be as follows:.
According to experiment 1, mass of Mg ribbon is 0.020 g, volume of acid used is 10.0 mL and volume of
The density of
Putting the values,
Molar mass of
From the balanced chemical reaction, 1 mol of hydrogen gas is produced from 1 mol of Mg thus, number of moles of Mg required to produce
The mass of Mg is 0.020 g and molar mass of Mg is 24.305 g/mol thus, number of moles of Mg will be:.
Since, number of moles of Mg required is
(c)
Interpretation:
The limiting reactant in experiment 3 should be determined..
Concept introduction:.
The number of moles of a substance is related to mass and molar mass as follows:.
Here,mis mass andMis molar mass of the substance..
The density of solution can be calculated as follow:.
Here, m is mass and V is volume.
Answer to Problem 74QAP
Mg is limiting reactant.
Explanation of Solution
The balanced chemical reaction will be as follows:.
According to experiment 3, mass of Mg ribbon is 0.080 g, volume of acid used is 10.0 mL and volume of
The density of
Putting the values,
Molar mass of
From the balanced chemical reaction, 1 mol of hydrogen gas is produced from 1 mol of Mg thus, number of moles of Mg required to produce
The mass of Mg is 0.080 g and molar mass of Mg is 24.305 g/mol thus, number of moles of Mg will be:.
Since, number of moles of Mg required is
(d)
Interpretation:
The limiting reactant in experiment 6 should be determined..
Concept introduction:.
The number of moles of a substance is related to mass and molar mass as follows:.
Here,mis mass andMis molar mass of the substance..
The density of solution can be calculated as follow:.
Here, m is mass and V is volume.
Answer to Problem 74QAP
Acid is limiting reactant.
Explanation of Solution
The balanced chemical reaction will be as follows:.
According to experiment 6, mass of Mg ribbon is 0.200 g, volume of acid used is 10.0 mL and volume of
The density of
Putting the values,
Molar mass of
From the balanced chemical reaction, 1 mol of hydrogen gas is produced from 1 mol of Mg thus, number of moles of Mg required to produce
The mass of Mg is 0.200 g and molar mass of Mg is 24.305 g/mol thus, number of moles of Mg will be:.
Since, number of moles of Mg required is
(e)
Interpretation:
The experiment that uses stoichiometric amounts of each reactant should be determined..
Concept introduction:.
The number of moles of a substance is related to mass and molar mass as follows:.
Here,mis mass andMis molar mass of the substance..
The density of solution can be calculated as follow:.
Here, m is mass and V is volume.
Answer to Problem 74QAP
Experiment 4.
Explanation of Solution
According to balance chemical reaction, 1 mol of Mg gives 1 mol of hydrogen gas thus, the experiment in which same number of moles of Mg reacts with acid to form hydrogen gas that experiment uses stoichiometric amounts of each reactant..
This cannot be experiment 1, 3 and 6 because ratio of number of moles of Mg and hydrogen gas is not 1:1 in these experiments..
Check experiment 2: mass of Mg is 0.040 g and molar mass of Mg is 24.305 g/mol thus, number of mol of Mg will be:
The volume of
The density of
Putting the values,
Molar mass of
The number of moles of Mg and hydrogen gas is not same thus, it is not experiment 2..
Check experiment 4: mass of Mg is 0.120 g and molar mass of Mg is 24.305 g/mol thus, number of mol of Mg will be:
The volume of
The density of
Putting the values,
Molar mass of
The number of moles of Mg and hydrogen gas is approximately same thus, it is experiment 4..
Check experiment 5: mass of Mg is 0.160 g and molar mass of Mg is 24.305 g/mol thus, number of mol of Mg will be:
The volume of
The density of
Putting the values,
Molar mass of
The number of moles of Mg and hydrogen gas is not same thus, it is not experiment 4..
Therefore, experiment 4 uses stoichiometric amounts of each reactant.
(f)
Interpretation:
The volume of the gas for 0.300 g and 0.010 g of Mg ribbon should be calculated.
Concept introduction:.
The number of moles of a substance is related to mass and molar mass as follows:.
Here,mis mass andMis molar mass of the substance..
The density of solution can be calculated as follow:.
Here, m is mass and V is volume.
Answer to Problem 74QAP
The volume of hydrogen gas produced from 0.120 g of Mg and 0.010 g of Mg is 122 mL and 11.32 mL respectively.
Explanation of Solution
The graph between mass of Mg ribbon and volume of hydrogen gas is as follows:.
![OWLv2 with Student Solutions Manual eBook for Masterton/Hurley's Chemistry: Principles and Reactions, 8th Edition, [Instant Access], 4 terms (24 months), Chapter 3, Problem 74QAP , additional homework tip 3](https://content.bartleby.com/tbms-images/9781305079373/Chapter-3/images/html_79373-3-74qap_3.png)
According to the graph, above the mass of Mg 0.120 g, the volume of hydrogen gas becomes constant at 122 mL thus, the volume of hydrogen gas produced if 0.120 g of Mg is burned will be 122 mL.
Considering only the straight line in the graph,.
| Experiment | Mass of Mg ribbon (g) | Volume of acid used (mL) | Volume of hydrogen gas (mL) |
| 1 | 0.020 | 10.0 | 21 |
| 2 | 0.040 | 10.0 | 42 |
| 3 | 0.080 | 10.0 | 82 |
| 4 | 0.120 | 10.0 | 122 |
The plot will be as follows:.
![OWLv2 with Student Solutions Manual eBook for Masterton/Hurley's Chemistry: Principles and Reactions, 8th Edition, [Instant Access], 4 terms (24 months), Chapter 3, Problem 74QAP , additional homework tip 4](https://content.bartleby.com/tbms-images/9781305079373/Chapter-3/images/html_79373-3-74qap_4.png)
Comparing this with equation of straight line
For the mass of ribbon 0.010 g, the volume of hydrogen gas can be calculated as follows:.
Therefore, the volume of hydrogen gas is 11.32 mL.
Want to see more full solutions like this?
Chapter 3 Solutions
OWLv2 with Student Solutions Manual eBook for Masterton/Hurley's Chemistry: Principles and Reactions, 8th Edition, [Instant Access], 4 terms (24 months)
- 4.69 The pictures below show a molecular-scale view of a chemical reaction between H2 and CO to produce methanol, CH3OH. The box on the left represents the reactants at the instant of mixing, and the box on the right shows what is left once the reaction has gone to completion. Was there a limiting reactant in this reaction? If so, what was it? Write a balanced chemical equation for this reaction. As usual, your equation should use the smallest possible whole number coefficients for all substances.arrow_forwardWrite an equation from the following description: reactants are gaseous NH3 and O2, products are gaseous NO2 and liquid H2O, and the stoichiometric coefficients are 4, 7, 4, and 6, respectively.arrow_forwardFig. 5-5 illustrates a schematic diagram of a combustion device used to analyze organic compounds. Given that a certain amount of a compound containing carbon, hydrogen, and oxygen is combusted in this device, explain how the data relating to the mass of CO2 produced and the mass of H2O produced can be manipulated to determine the empirical formula.arrow_forward
- 3.115 The average person exhales 1.0 kg of carbon dioxide in a day. Describe how you would estimate the number of CO2 molecules exhaled per breath for this average person.arrow_forwardMany cereals are made with high moisture content so that the cereal can be formed into various shapes before it is dried. A cereal product containing 58% H2O by mass is produced at the rate of 1000. kg/h. What mass of water must be evaporated per hour if the final product contains only 20.% water?arrow_forward3.105 Nitric acid is often sold and transported as a concentrated 16 M aqueous solution. How many gallons of such a solution would be needed to contain the roughly 2.1109 pounds of HNO3 produced annually in the United States?arrow_forward
- Many cereals are made with high moisture content so that the cereal can be formed into various shapes before it is dried. A cereal product containing 58% H2O by mass is produced at the rate of 1000. kg/h. What mass of water must be evaporated per hour if the final product contains only 20.% water?arrow_forward4.19 How many metric tons of carbon are required to react with 7.83 metric tons of Fe2O3 according to the following reaction? 2Fe2O3+3C3CO2+4Fe How many metric tons of iron are produced?arrow_forwardA power plant is driven by the combustion of a complex fossil fuel having the formula C11H7S. Assume the air supply is composed of only N2 and O2 with a molar ratio of 3.76:1.00, and the N2 remains unreacted. In addition to the water produced, the fuels C is completely combusted to CO2 and its sulfur content is converted to SO2. In order to evaluate gases emitted at the exhaust stacks for environmental regulation purposes, the nitrogen supplied with the air must also be included in the balanced reactions. a Including the N2 supplied m the air, write a balanced combustion equation for the complex fuel assuming 100% stoichiometric combustion (i.e., when there is no excess oxygen in the products and the only C-containing product is CO2). Except in the case of N2, use only integer coefficients. b Including N2 supplied in the air, write a balanced combustion equation for the complex fuel assuming 120% stoichiometric combustion (i.e., when excess oxygen is present in the products and the only C-containing product is CO2). Except in the case of use only integer coefficients c Calculate the minimum mass (in kg) of air required to completely combust 1700 kg of C11H7S. d Calculate the air/fuel mass ratio, assuming 100% stoichiometric combustion. e Calculate the air/fuel mass ratio, assuming 120% stoichiometric combustion.arrow_forward
- A titanium ore contains rutile (TiO2) plus some iron oxide and silica. When it is heated with carbon in the presence of chlorine, titanium tetrachloride, TiCl4, is formed. TiO2(s)+C(s)+2Cl2(g)TiCl4(g)+CO2(g) Titanium tetrachloride, a liquid, can be distilled from the mixture. If 35.4 g of titanium tetrachloride is recovered from 18.1 g of crude ore, what is the mass percentage of TiO2 in the ore (assuming all TiO2 reacts)?arrow_forward4-52 The molecular weight of hemoglobin is about 68,000 amu. What is the mass in grams of a single molecule of hemoglobin?arrow_forwardFor this reaction, fill in the table with the indicated quantities for the balanced equation. 4 NH3(g) + 5 O2(g) → 4 NO(g) + 6 H2O(g)arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





