
Organic Chemistry, Books a la Carte Edition (8th Edition)
8th Edition
ISBN: 9780134074580
Author: Bruice, Paula Yurkanis
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3, Problem 59P
a. How many primary carbons does each of the following compounds have?
b. How many secondary carbons does each one have?
c. How many tertiary carbons does each one have?
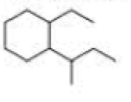
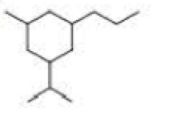
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Predict the major organic product(s), if any, of the following reactions. Assume all reagents are in excess unless otherwise indicated.
How many signals would you expect to find in the 1 H NMR spectrum of each given compound?
Part 1 of 2
2
Part 2 of 2
HO
5
☑
Х
IIIIII*****
§
A carbonyl compound has a molecular
ion with a m/z of 86. The mass spectra
of this compound also has a base peak
with a m/z of 57. Draw the correct
structure of this molecule.
Drawing
Chapter 3 Solutions
Organic Chemistry, Books a la Carte Edition (8th Edition)
Ch. 3.1 - Name each of the following:Ch. 3.1 - Draw the structure of a compound with molecular...Ch. 3.1 - Draw the structures and name the four...Ch. 3.1 - Prob. 6PCh. 3.1 - Draw the structure for each of the following: a....Ch. 3.1 - Name the following compounds: a. CH3OCH2CH3 b....Ch. 3.2 - Prob. 9PCh. 3.2 - Draw the structure for each of the following: a....Ch. 3.2 - Give each substituent on the nine-carbon chain a...Ch. 3.2 - Prob. 14P
Ch. 3.3 - What is each compounds systematic name?Ch. 3.3 - Prob. 16PCh. 3.3 - Prob. 17PCh. 3.3 - Prob. 18PCh. 3.3 - Prob. 19PCh. 3.4 - Give two names for each of the following alkyl...Ch. 3.4 - Prob. 21PCh. 3.5 - a. What is each ethers systematic name? 1....Ch. 3.6 - Give each of the following a systematic name and...Ch. 3.6 - Draw the structures of a homologous series of...Ch. 3.6 - Prob. 25PCh. 3.6 - Prob. 26PCh. 3.7 - Prob. 27PCh. 3.7 - Are the following compounds primary, secondary, or...Ch. 3.7 - Draw condensed and skeletal structures for each of...Ch. 3.7 - For each of the following, give the systematic...Ch. 3.8 - Predict the approximate size of the following bond...Ch. 3.9 - Prob. 32PCh. 3.9 - Prob. 33PCh. 3.9 - Prob. 34PCh. 3.9 - Rank the following compounds from highest boiling...Ch. 3.9 - Rank the compounds in each set from highest...Ch. 3.10 - In which solvent would cyclohexane have the lowest...Ch. 3.10 - Prob. 38PCh. 3.10 - Prob. 39PCh. 3.11 - a. Draw all the staggered and eclipsed conformers...Ch. 3.11 - Prob. 41PCh. 3.11 - Using Newman projections, draw the most stable...Ch. 3.12 - The bond angles in a regular polygon with n sides...Ch. 3.12 - Prob. 44PCh. 3.13 - Draw 1,2,3,4,5,6-hexachlorocydohexane with a. all...Ch. 3.14 - Using the data in Table 3.9, calculate the...Ch. 3.14 - The chair conformer of fluorocyclohexane is 0.25...Ch. 3.15 - Prob. 48PCh. 3.15 - Which has a higher percentage of the...Ch. 3.15 - a. Draw the more stable chair conformer of...Ch. 3.15 - For each of the following disubstituted...Ch. 3.15 - a. Draw Newman projections of the two conformers...Ch. 3.15 - a. Calculate the energy difference between the two...Ch. 3 - a. How many hydrogen does an alkene with 17...Ch. 3 - Draw the structure of octane and isooctaneCh. 3 - Draw a condensed structure and a skeletal...Ch. 3 - Prob. 56PCh. 3 - a. What is each compounds systematic name? b. Draw...Ch. 3 - Which of the following represents a cis isomer?Ch. 3 - a. How many primary carbons does each of the...Ch. 3 - Which of the following conformers of isobutyl...Ch. 3 - Draw a skeletal structure for an alkane that has...Ch. 3 - What is each compounds systematic name? a....Ch. 3 - Which bus a. the higher boiling point:...Ch. 3 - a. Draw Newman projections of the two conformers...Ch. 3 - Ansaid and Motrin belong to the group of drugs...Ch. 3 - Prob. 66PCh. 3 - A student was given the structural formulas of...Ch. 3 - Which of the following conformers has the highest...Ch. 3 - Prob. 69PCh. 3 - Draw skeletal structures for the following: a....Ch. 3 - For rotation about the C-3C-4 bond of...Ch. 3 - Prob. 72PCh. 3 - What is each compounds systematic name? a. b. c....Ch. 3 - Draw the two chair conformers for each of the...Ch. 3 - Why are lower molecular weight alcohols more...Ch. 3 - a. Draw a potential energy diagram for rotation...Ch. 3 - For each of the following compound, determine...Ch. 3 - How many ethers have molecular formula C5H12O?...Ch. 3 - Draw the most stable conformer of the following...Ch. 3 - What is each compounds systematic name?Ch. 3 - Calculate the energy difference between the two...Ch. 3 - The most stable from of glucose (blood sugar) is a...Ch. 3 - What is each compound s systematic name?Ch. 3 - Explain the following: a. 1-Hexanol has a higher...Ch. 3 - One of the chair conformers of cis-...Ch. 3 - Bromine is a larger atom than chlorine, but the...Ch. 3 - Name the following compounds:Ch. 3 - Prob. 88PCh. 3 - Using the data obtained in Problem 85, calculate...Ch. 3 - Draw the conformers for the following...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can you draw this using Lewis dot structures and full structures in the same way they are so that I can better visualize them and then determine resonance?arrow_forwardSynthesize the following compound from cyclohexanol, ethanol, and any other needed reagentsarrow_forwardFor a titration of 20.00 mL of 0.0500 M H2SO4 with 0.100 M KOH, calculate the pH at each of the following volume of KOH used in the titration: 1) before the titration begin; 2) 10.00 mL; 3) 20.00 mL; 4) 30.00 mL. Ka2 = 1.20×10-2 for H2SO4.arrow_forward
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s) Be sure to account for all bond-breaking and bond-making steps Problem 73 of 10 Drawing Amows ro HO Donearrow_forward12. Synthesize the following target molecules (TMs) using the specified starting materials. .CI a) HO3S SM TM b) HO- SMarrow_forwardFor a titration of 20.00 mL of 0.0500 M H2SO4 with 0.100 M KOH, calculate the pH at each of the following volume of KOH used in the titration: 1) before the titration begin; 2) 10.00 mL; 3) 20.00 mL; 4) 30.00 mL. Ka2 = 1.20×10-2 for H2SO4.arrow_forward
- Write the systematic name of each organic molecule: structure name show work. don't give Ai generated solutionarrow_forwardShow work with explanation needed. Don't give Ai generated solutionarrow_forwardA Elschboard Part of SpeechT-D Alt Leaming App app.aktiv.com Curved arrows are used to illustrate the flow of electrons. Using the provided resonance structures, draw the curved electron- pushing arrows to show the interconversion between resonance hybrid contributors. Be sure to account for all bond-breaking and bond-making steps. Include all lone pairs and formal charges in the structures. Problem 45 of 10 I Select to Add Arrows N Please selarrow_forward
- So I'm working on molecular geometry. Can you help me with this stuff here and create three circles: one that's 120, one that’s 180, and one that’s 109.5?arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Problem 164 of N Select to Add Arrows CHI CH 1 1 1 Parrow_forwardusing these can you help me , I guess convert them to lewis dit structures or full drawn out skeletal and I guess is that what would help me depict the bond angle.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY