
Concept explainers
Answer the following for water samples A and B Shown in diagrams: (3.1, 3.2, 3.3, 3.5)
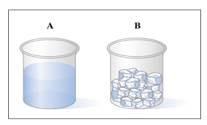
a. In which sample (A or B) does the water have its own shape?
b. Which diagram (1 or 2 or 3) represents the arrangement of particles in water sample A?
c. Which diagram (1 or 2 or 3) represents arrangement of particles in water B?
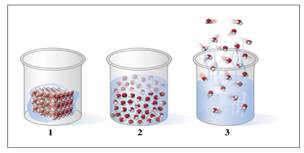
Answer the following for diagrams 1, 2, and 3:
d. The
e. The motion of the particles is slowest in diagram .
f. The arrangement of particular is farther apart in diagram .
g. The particles fill the volume of the container in diagram .
h. If the water in diagram 2 has a mass of 19 g and a temperature of 45 °C, how much heat, in kilojoules, is removed to cool the liquid to 0 °C?

An energy bar contains carbohydrate, fat, and protein.
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
- true or false The equilibrium constant for this reaction is 0.20. N2O4(g) ⇔ 2NO2(g) Based on the above, the equilibrium constant for the following reaction is 5. 4NO2(g) ⇔ 2N2O4(g)arrow_forwardtrue or false The equilibrium constant for this reaction is 0.20. N2O4(g) ⇔ 2NO2(g) Based on the above, the equilibrium constant for the following reaction is 0.4. 2N2O4(g) ⇔ 4NO2(g)arrow_forwardtrue or false Using the following equilibrium, if heat is added the equilibrium will shift toward the reactants. N2(g) + 3H2(g) ⇔ 2NH3(g) + heatarrow_forward
- True or False Using the following equilibrium, if heat is added the equilibrium will shift toward the products. N2O4(g) + heat ⇔ 2NO2(g)arrow_forwardtrue or false Using the following equilibrium, if solid carbon is added the equilibrium will shift toward the products. C(s) + CO2(g) ⇔ 2CO(g)arrow_forwardProvide the complete mechanism for the reaction below. You must include appropriate arrows,intermediates, and formal charges. Please also provide a reason to explain why the 1,4-adduct is preferred over the 1,3-adduct.arrow_forward
- Which of the following pairs are resonance structures of one another? I. III. || III IV + II. :0: n P !༠ IV. EN: Narrow_forwardPredict the major organic product(s) and byproducts (either organic or inorganic) for thefollowing reactions.arrow_forwardA 8.25 g sample of aluminum at 55°C released 2500 J of heat. The specific heat of aluminum is 0.900 J/g°C. The density of aluminum is 2.70 g/mL. Calculate the final temperature of the aluminum sample in °C.arrow_forward
