
EBK CAMPBELL BIOLOGY IN FOCUS
2nd Edition
ISBN: 8220101459299
Author: Reece
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3, Problem 2TYU
Which
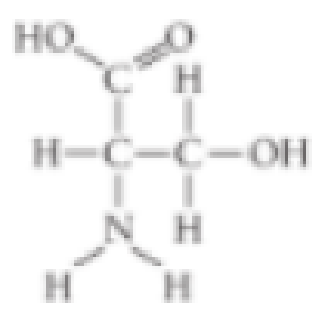
- A. carboxyl
- B. sulfhydryl
- C. hydroxyl
- D. amino
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The current nutrition labelling regulation in Hong Kong requires food manufacturer to list E+7 information on the package of pre-packaged food products. Do you think that more nutrients, such as calcium and cholesterol, shall be included?
View
History Bookmarks
Window
Help
Quarter
cements
ents
ons
(17) YouTube
Which amino acids
would you expect to find marked on the alpha helix?
canvas.ucsc.edu
ucsc Complaint and Grievance Process - Academic Personnel
pach
orations
| | | | | | | |
| | | | | | | |
000000
000000000
| | | | | | | | | | |
| | | | | | | | | | |
00000000
scope
vious
De
48
12.415
KATPM
FEB
3
F1
F2
80
F3
a
F4
F5
2
#
3
$
85
%
tv N
A
の
Mon Feb 3 10:24 PM
Lipid
bilayer
Submit Assignment
Next >
ZOOM
<
Å
DII
8
བ
བ
F6
16
F7
F8
F9
F10
34
F11
F12
&
*
(
6
7
8
9
0
+ 11
WERTY U
{
0
}
P
delete
Different species or organisms research for ecology
Chapter 3 Solutions
EBK CAMPBELL BIOLOGY IN FOCUS
Ch. 3.1 - How are gasoline and fat chemically similar?Ch. 3.1 - Which molecules in Figure 3.4a re isomers? For...Ch. 3.1 - Prob. 3CCCh. 3.1 - Prob. 4CCCh. 3.2 - How many molecules of water are needed to...Ch. 3.2 - WHAT IF? Suppose you eat a serving of fish. What...Ch. 3.3 - Write the formula for a monosaccharide that has...Ch. 3.3 - A dehydration reaction joins two glucose molecules...Ch. 3.3 - WHAT IF? After a cow is given antibiotics to treat...Ch. 3.4 - Compare the structure of a fat (triglyceride) with...
Ch. 3.4 - Why are human sex hormones considered lipids?Ch. 3.4 - Prob. 3CCCh. 3.5 - Why does a denatured protein no longer function...Ch. 3.5 - What parts of a polypeptide participate in the...Ch. 3.5 - WHAT IF? Where would you expect a polypeptide...Ch. 3.6 - DRAW IT Go to Figure 3.27a and, for the top three...Ch. 3.6 - Prob. 2CCCh. 3.7 - How would sequencing the entire genome of an...Ch. 3.7 - Given the function of DNA, why would you expect...Ch. 3 - Prob. 1TYUCh. 3 - Which functional group is not present in this...Ch. 3 - MAKE CONNECTIONS Which chemical group is most...Ch. 3 - Prob. 4TYUCh. 3 - Which of the following statements concerning...Ch. 3 - The structural level of a protein least a fleeted...Ch. 3 - Enzymes that break down DNA catalyze the...Ch. 3 - Prob. 8TYUCh. 3 - The molecular formula for glucose is C6H12O6. What...Ch. 3 - Construct a table that organizes the following...Ch. 3 - Prob. 11TYUCh. 3 - Prob. 12TYUCh. 3 - FOCUS ON ORGANIZATION Proteins, which have diverse...Ch. 3 - Prob. 14TYUCh. 3 - SYNTHESIZE YOUR KNOWLEDGE Given that the function...
Additional Science Textbook Solutions
Find more solutions based on key concepts
True or false? Some trails are considered vestigial because they existed long ago.
Biological Science (6th Edition)
1. Rub your hands together vigorously. What happens? Discuss the energy transfers and transformations that take...
College Physics: A Strategic Approach (3rd Edition)
Determine [OH], [H+], and the pH of each of the following solutions. a. 1.0 M KCl b. 1.0 M KC2H3O2
Chemistry
Single penny tossed 20 times and counting heads and tails: Probability (prediction): _______/20 heads ________/...
Laboratory Manual For Human Anatomy & Physiology
How does the removal of hydrogen atoms from nutrient molecules result in a loss of energy from the nutrient mol...
SEELEY'S ANATOMY+PHYSIOLOGY
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- What is the result of the following gram stain: positive ○ capsulated ○ acid-fast ○ negativearrow_forwardWhat type of stain is the image below: capsule stain endospore stain gram stain negative stain ASM MicrobeLibrary.org Keplingerarrow_forwardWhat is the result of the acid-fast stain below: Stock Images by Getty Images by Getty Images by Getty Images by Getty Image Getty Images St Soy Getty Images by Getty Images by Getty Images Joy Getty encapsulated O endosporulating negative ○ positivearrow_forward
- You have a stock vial of diligence 75mg in 3ml and need to draw up a dose of 50mg for your patient.how many mls should you draw up to give this dosearrow_forwardYou are recquired to administer 150mg hydrocortisone intravenously,how many mls should you give?(stock =hydrocortisone 100mg in 2mls)arrow_forwardIf someone was working with a 50 MBq F-18 source, what would be the internal and external dose consequences?arrow_forward
- We will be starting a group project next week where you and your group will research and ultimately present on a current research article related to the biology of a pathogen that infects humans. The article could be about the pathogen itself, the disease process related to the pathogen, the immune response to the pathogen, vaccines or treatments that affect the pathogen, or other biology-related study about the pathogen. I recommend that you choose a pathogen that is currently interesting to researchers, so that you will be able to find plenty of articles about it. Avoid choosing a historical disease that no longer circulates. List 3 possible pathogens or diseases that you might want to do for your group project.arrow_forwardnot use ai pleasearrow_forwardDNK dagi nukleotidlar va undan sintezlangan oqsildagi peptid boglar farqi 901 taga teng bo'lib undagi A jami H boglardan 6,5 marta kam bo'lsa DNK dagi jami H bog‘lar sonini topingarrow_forward
- One of the ways for a cell to generate ATP is through the oxidative phosphorylation. In oxidative phosphorylation 3 ATP are produced from every one NADH molecule. In respiration, every glucose molecule produces 10 NADH molecules. If a cell is growing on 5 glucose molecules, how much ATP can be produced using oxidative phosphorylation/aerobic respiration?arrow_forwardIf a cell is growing on 5 glucose molecules, how much ATP can be produced using oxidative phosphorylation/aerobic respiration?arrow_forwardHow do i know which way the arrows go?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
 Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College

Biology (MindTap Course List)
Biology
ISBN:9781337392938
Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:Cengage Learning

Biology Today and Tomorrow without Physiology (Mi...
Biology
ISBN:9781305117396
Author:Cecie Starr, Christine Evers, Lisa Starr
Publisher:Cengage Learning

Human Heredity: Principles and Issues (MindTap Co...
Biology
ISBN:9781305251052
Author:Michael Cummings
Publisher:Cengage Learning

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:9781305389892
Author:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:Cengage Learning


Anatomy & Physiology
Biology
ISBN:9781938168130
Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:OpenStax College
Macromolecules | Classes and Functions; Author: 2 Minute Classroom;https://www.youtube.com/watch?v=V5hhrDFo8Vk;License: Standard youtube license