
Pearson eText Principles of Human Physiology -- Instant Access (Pearson+)
6th Edition
ISBN: 9780135213001
Author: Cindy Stanfield
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3, Problem 28E
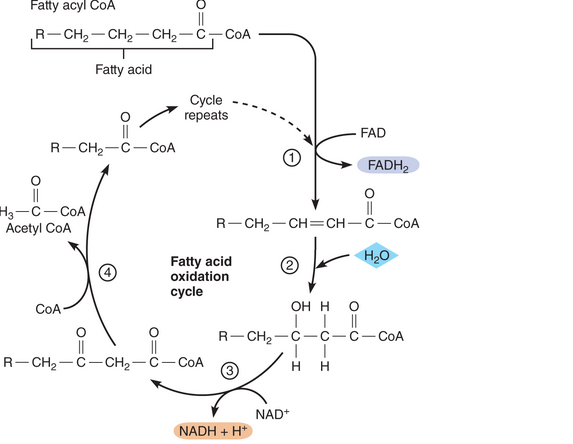
As you know, fatty acids can be oxidized to provide energy for making ATP. This process begins with a set of reactions known as the fatty acid oxidation cycle, or beta oxidation. Before entering the cycle, a fatty acid reacts with coenzyme A to form a molecule called fatty acyl CoA. This molecule then enters the cycle and continues to go through it until all its carbons have been eliminated, as shown on the following page. From your examination of this diagram, answer the following questions for a 1 2-carbon fatty acid.
- How many acetyl CoA molecules will be produced by beta oxidation of this fatty acid? How many ATP molecules can be produced from each acetyl CoA molecule that goes through the Krebs cycle and oxidative phosphorylation?
- How many NAD+ and FAD molecules will be reduced? (Hint: How many bonds must be broken to split a 12-carbon fatty acid into acetyl CoA molecules?)
- How many ATP molecules will be produced per each NADH + H+ and each FADH2 molecule that provides electrons to the electron transport system?
- It takes two ATP molecules to get the cycle started. Based on this information and your answers to the previous questions, how many ATP molecules can be generated from a 1 2-carbon fatty acid?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
C
MasteringHealth MasteringNu ×
session.healthandnutrition-mastering.pearson.com/myct/itemView?assignment ProblemID=17396416&attemptNo=1&offset=prev
10. Your instructor will give you 2 amino acids during the activity session (video 2-7.
A. First color all the polar and non-polar covalent bonds in the R groups of your 2 amino acids
using the same colors as in #7. Do not color the bonds in the backbone of each amino acid.
B. Next, color where all the hydrogen bonds, hydrophobic interactions and ionic bonds could
occur in the R group of each amino acid. Use the same colors as in #7. Do not color the bonds
in the backbone of each amino acid.
C. Position the two amino acids on the page below in an orientation where the two R groups
could bond together. Once you are satisfied, staple or tape the amino acids in place and label
the bond that you formed between the two R groups.
- Polar covalent Bond - Red
- Non polar Covalent boND- yellow
- Ionic BonD - PINK
Hydrogen Bonn - Purple
Hydrophobic interaction-green
O=C-N
H
I.
H
HO
H
=O
CH2
C-C-N
HICK
H
HO
H
CH2
OH
H₂N
C = O
Find the dental formula and enter it in the following format:
I3/3 C1/1 P4/4 M2/3 = 42 (this is not the correct number, just the correct format)
Please be aware: the upper jaw is intact (all teeth are present). The bottom jaw/mandible is not intact. The front teeth should include 6 total rectangular teeth (3 on each side) and 2 total large triangular teeth (1 on each side).
Chapter 3 Solutions
Pearson eText Principles of Human Physiology -- Instant Access (Pearson+)
Ch. 3.1 - What is the primary distinction between an...Ch. 3.1 - Describe in general terms what occurs in...Ch. 3.2 - In an exergonic reaction, which has more...Ch. 3.2 - Which factor determines the direction of a...Ch. 3.2 - Prob. 3.2.3QCCh. 3.2 - What is activation energy? How does it affect a...Ch. 3.3 - Prob. 3.3.1QCCh. 3.3 - How is the rate of an enzymatic reaction affected...Ch. 3.3 - What is the primary distinction between allosteric...Ch. 3.5 - Prob. 3.4.1QC
Ch. 3.5 - Prob. 3.4.2QCCh. 3.5 - Prob. 3.4.3QCCh. 3.6 - Prob. 1CTQCh. 3.6 - Prob. 2CTQCh. 3.6 - Prob. 3CTQCh. 3.6 - Prob. 3.5.1QCCh. 3.6 - Prob. 3.5.2QCCh. 3.6 - Prob. 3.5.3QCCh. 3.6 - Prob. 3.5.4QCCh. 3.7 - Prob. 3.6.1QCCh. 3.7 - Prob. 3.6.2QCCh. 3.7 - Prob. 3.6.3QCCh. 3 - Prob. 1ECh. 3 - Prob. 2ECh. 3 - Prob. 3ECh. 3 - Prob. 4ECh. 3 - Prob. 5ECh. 3 - Prob. 6ECh. 3 - Prob. 7ECh. 3 - Prob. 8ECh. 3 - Prob. 9ECh. 3 - Prob. 10ECh. 3 - The removal of hydrogen atoms from a molecule is...Ch. 3 - Prob. 12ECh. 3 - Prob. 13ECh. 3 - Prob. 14ECh. 3 - Prob. 15ECh. 3 - Prob. 16ECh. 3 - Prob. 17ECh. 3 - Prob. 18ECh. 3 - Prob. 19ECh. 3 - Prob. 20ECh. 3 - Prob. 21ECh. 3 - Prob. 22ECh. 3 - Prob. 23ECh. 3 - Compare and contrast the mechanisms of...Ch. 3 - Explain how the conversion of pyruvate to lactate...Ch. 3 - Prob. 26ECh. 3 - Prob. 27ECh. 3 - As you know, fatty acids can be oxidized to...Ch. 3 - Prob. 29E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- 12. Calculate the area of a circle which has a radius of 1200 μm. Give your answer in mm² in scientific notation with the correct number of significant figures.arrow_forwardDescribe the image quality of the B.megaterium at 1000X before adding oil? What does adding oil do to the quality of the image?arrow_forwardWhich of the follwowing cells from this lab do you expect to have a nucleus and why or why not? Ceratium, Bacillus megaterium and Cheek epithelial cells?arrow_forward
- 14. If you determine there to be debris on your ocular lens, explain what is the best way to clean it off without damaging the lens?arrow_forward11. Write a simple formula for converting mm to μm when the number of mm's is known. Use the variable X to represent the number of mm's in your formula.arrow_forward13. When a smear containing cells is dried, the cells shrink due to the loss of water. What technique could you use to visualize and measure living cells without heat-fixing them? Hint: you did this technique in part I.arrow_forward
- 10. Write a simple formula for converting μm to mm when the number of μm's are known. Use the variable X to represent the number of um's in your formula.arrow_forward8. How many μm² is in one cm²; express the result in scientific notation. Show your calculations. 1 cm = 10 mm; 1 mm = 1000 μmarrow_forwardFind the dental formula and enter it in the following format: I3/3 C1/1 P4/4 M2/3 = 42 (this is not the correct number, just the correct format) Please be aware: the upper jaw is intact (all teeth are present). The bottom jaw/mandible is not intact. The front teeth should include 6 total rectangular teeth (3 on each side) and 2 total large triangular teeth (1 on each side).arrow_forward
- Answer iarrow_forwardAnswerarrow_forwardcalculate the questions showing the solution including variables,unit and equations all the questiosn below using the data a) B1, b) B2, c) hybrid rate constant (1) d) hybrid rate constant (2) e) t1/2,dist f) t1/2,elim g) k10 h) k12 i) k21 j) initial concentration (C0) k) central compartment volume (V1) l) steady-state volume (Vss) m) clearance (CL) AUC (0→10 min) using trapezoidal rule n) AUC (20→30 min) using trapezoidal rule o) AUCtail (AUC360→∞) p) total AUC (using short cut method) q) volume from AUC (VAUC)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning

Concepts of Biology
Biology
ISBN:9781938168116
Author:Samantha Fowler, Rebecca Roush, James Wise
Publisher:OpenStax College

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Human Physiology: From Cells to Systems (MindTap ...
Biology
ISBN:9781285866932
Author:Lauralee Sherwood
Publisher:Cengage Learning



Anaerobic Respiration; Author: Bozeman Science;https://www.youtube.com/watch?v=cDC29iBxb3w;License: Standard YouTube License, CC-BY