
Concept explainers
(a)
Interpretation:
The given diagram has to be classified as a mixture, an element or a compound.
The given diagram is,

Figure 1
Concept Introduction:
Matter can be classified into two type’s namely pure substance and mixture.
Pure substance: A single component that has a constant composition, irrespective of the
Example: Water, sugar etc.
A pure substance can be classified into an element and a compound.
Element: A pure substance, which cannot be broken down into smaller substances by a
Example: Hydrogen gas, Magnesium ribbon and copper wire etc.
Compound: A pure substance that is formed by combination of two or more elements by chemical process is called as a compound. Example: Sodium chloride is a compound because it is formed from elements sodium and chlorine.
Mixture: A mixture consists of more than one substance and the composition of a mixture is dependent on the sample. The separation of mixture into its components can be done by physical changes.
(b)
Interpretation:
The given diagram has to be classified as a mixture, an element or a compound.
The given diagram is,
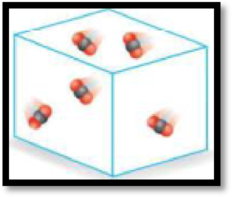
Figure 2
Concept Introduction:
Refer to part (a).
(c)
Interpretation:
The given diagram has to be classified as a mixture, an element or a compound.
The given diagram is,

Figure 3
Concept Introduction:
Refer to part (a).
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
FOUNDATIONS OF COLLEGE CHEM +KNEWTONALTA
- A mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forwardPLEASE HELP ! URGENT!arrow_forward
- Identify priority of the substituents: CH3arrow_forwardHow many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





