
(a)
Interpretation:
The critical temperature and critical pressure of a gasneeds to be defined.
Concept introduction:
Critical temperature is the temperature at which the vapor of the substance cannot be liquefied even if the pressure is applied. Critical pressure is the pressure at which the gas can be liquefied at its critical temperature.
Answer to Problem 29.1QAP
The temperature at which a substance cannot be liquefied from its vapor form is termed as critical temperature. The pressure at which the gas can be liquefied at its critical temperature is termed as critical pressure.
Explanation of Solution
When gases are compressed at suitable temperature, it gets converted to liquids. If the temperature is increased, the kinetic energy of the gas molecules increases making it more difficult for the gas to liquefy. Even if the pressure is increased, it does not have an impact. Hence, this temperature at which the gas cannot be liquefied is termed as critical temperature.
Similarly, the pressure that is required to liquefy a gas at its critical temperature, is said to be critical pressure.
(b)
Interpretation:
Supercritical fluid needs to be defined.
Concept introduction:
A fluid that does not exist as liquid or gas above its critical temperature and pressure is said to be supercritical fluid. The special features of this fluid is that it can pass through solid like gas and dissolve materials as liquid does.
Answer to Problem 29.1QAP
Supercritical fluids are those that exist having their temperature and pressure beyond the critical point. Hence, it has low viscosity and high density. This does not allow for the substance to liquefy even if the pressure is increased.
Explanation of Solution
Fluids that does not have a distinct liquid or gaseous phase above its critical point is termed as supercritical fluid.
Upon observing the graph, it can be noticed that above the critical point, the substance cannot exist as a liquid or gas.
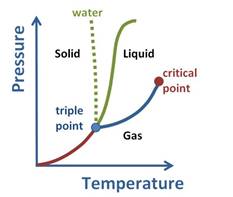
This is due to the fact that at higher temperatures, the kinetic energy of the molecules is high which will help overcome the intermolecular forces that are necessary for bringing about condensation or converting to liquid phase.
Besides this, at a high pressure, or pressure above critical pressure the substance cannot stay in gaseous state. There needs to be a balance between the two states.
Hence, the state at which the substance does not exist as liquid or gas is said to be supercritical fluid. It behaves such that it can pass through solid as gas does and dissolve materials as liquid does. This property makes it suitable as substitute for organic solvents. In other words, supercritical fluids behave as gas and as liquid.
Want to see more full solutions like this?
- Can I please get help with this?arrow_forwardUse the Henderson-Hasselbalch equation to calculate pH of a buffer containing 0.050M benzoic acidand 0.150M sodium benzoate. The Ka of benzoic acid is 6.5 x 10-5arrow_forwardA. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forward
- What is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardCan I please get help with this.arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forward
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





