
Concept explainers
(a)
Interpretation: The number of MOs in the given compound
Concept introduction:
Molecular orbital theory suggests that atomic orbitals of different atoms combines to create molecular orbitals.
Molecular orbitals can be constructed from linear combination of atomic orbitals.
Bonding orbotals are formed by the additive combination of atomic orbitals and the antibonding orbitals are formed by the substractive combination of atomic orbitals.
Antibonding orbital is a molecular orbital that results when two parallel atomic orbitals with opposite phases interact.
Antibonding orbitals have higher energy than the bonding molecular orbitals.
Ground state and and exited states are the positions with lower and higher energy respectively.
HOMO is a molecular orbital which is the abbrevation of Highest Occupied Molecular Orbital.
LUMO is also a molecular orbital which is the short form of Lowest Unoccupied Molecular Orbital.
If the lobes at the ends of the MO are in phase, then the MO is symmetric.
If the two lobes are out phase then the MO is antisymmetric.
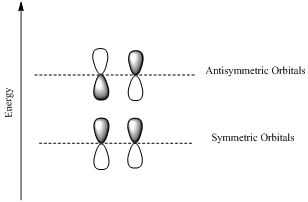
(b)
Interpretation: The designation of HOMO for the given molecule’s molecular orbital has to be given.
Concept introduction:
Molecular orbital theory suggests that atomic orbitals of different atoms combines to create molecular orbitals.
Molecular orbitals can be constructed from linear combination of atomic orbitals.
Bonding orbotals are formed by the additive combination of atomic orbitals and the antibonding orbitals are formed by the substractive combination of atomic orbitals.
Antibonding orbital is a molecular orbital that results when two parallel atomic orbitals with opposite phases interact.
Antibonding orbitals have higher energy than the bonding molecular orbitals.
Ground state and and exited states are the positions with lower and higher energy respectively.
HOMO is a molecular orbital which is the abbrevation of Highest Occupied Molecular Orbital.
LUMO is also a molecular orbital which is the short form of Lowest Unoccupied Molecular Orbital.
If the lobes at the ends of the MO are in phase, then the MO is symmetric.
If the two lobes are out phase then the MO is antisymmetric.
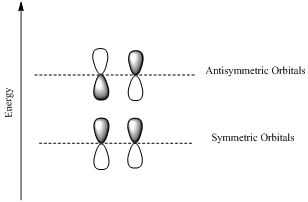
(c)
Interpretation: Number of nodes in the given molecule has to be given.
Concept introduction:
Molecular orbital theory suggests that atomic orbitals of different atoms combines to create molecular orbitals.
Molecular orbitals can be constructed from linear combination of atomic orbitals.
Bonding orbotals are formed by the additive combination of atomic orbitals and the antibonding orbitals are formed by the substractive combination of atomic orbitals.
Antibonding orbital is a molecular orbital that results when two parallel atomic orbitals with opposite phases interact.
Antibonding orbitals have higher energy than the bonding molecular orbitals.
Ground state and and exited states are the positions with lower and higher energy respectively.
HOMO is a molecular orbital which is the abbrevation of Highest Occupied Molecular Orbital.
LUMO is also a molecular orbital which is the short form of Lowest Unoccupied Molecular Orbital.
If the lobes at the ends of the MO are in phase, then the MO is symmetric.
If the two lobes are out phase then the MO is antisymmetric.
Node is the site with zero electron density.
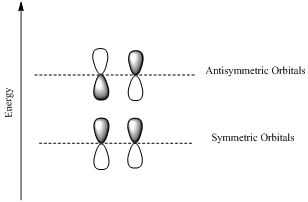
Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
Organic Chemistry (8th Edition)
- A covalent bond is the result of the a) b) c) d) e) overlap of two half-filled s orbitals overlap of a half-filled s orbital and a half-filled p orbital overlap of two half-filled p orbitals along their axes parallel overlap of two half-filled parallel p orbitals all of the abovearrow_forwardCan the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C T Add/Remove step X ноarrow_forwardWhich one of the following atoms should have the largest electron affinity? a) b) c) d) 으으 e) 1s² 2s² 2p6 3s¹ 1s² 2s² 2p5 1s² 2s² 2p 3s² 3p² 1s² 2s 2p 3s² 3p6 4s2 3ds 1s² 2s² 2p6arrow_forward
- All of the following are allowed energy levels except _. a) 3f b) 1s c) 3d d) 5p e) 6sarrow_forwardA student wants to make the following product in good yield from a single transformation step, starting from benzene. Add any organic reagents the student is missing on the left-hand side of the arrow, and any addition reagents that are necessary above or below the arrow. If this product can't be made in good yield with a single transformation step, check the box below the drawing area. Note for advanced students: you may assume that an excess of benzene is used as part of the reaction conditions. : ☐ + I X This product can't be made in a single transformation step.arrow_forwardPredict the major products of this organic reaction:arrow_forward
- Name the family to which each organic compound belongs. The first answer has been filled in for you. compound CH₂ || CH3-C-NH2 0 ။ CH3-C-CH₂ CH=O–CH=CH, CH₂ HO CH2-CH2-CH-CH3 family amine Darrow_forward1b. Br LOHarrow_forwardI would like my graphs checked please. Do they look right? Do I have iodine and persulfate on the right axis ?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
