
(a)
Interpretation:
Anticipating the isolation of the potentially aromatic hydrocarbon B, a group of chemists irradiated compound A with ultraviolet light. Compound C was obtained as a product instead of B.
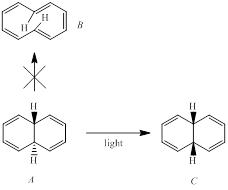
The reason for compound B might be expected to be unstable in spite of its cyclic array of 4n+2 π electrons is to be stated.
Concept introduction:
The stability of
(b)
Interpretation:
The formation of compound B is allowed by the pericyclic selection rules is to be explained.
Concept introduction:
Pericyclic reactions deal with electrons shift in a concerted manner. Moreover, in these reactions the breaking of reactant bonds and the formation of product bonds are formed at the same time.i.e., without the formation of intermediates. The word ‘pericyclic’ means around the cycle. Notably, the pericyclic reactions are classified as electrocyclic, cycloaddition and sigmatropic reactions based on the activation of reaction, the number of electrons involved and the stereochemistry outcome of the reactions. The selection rules of pericyclic reaction deal mostly on the
(c)
Interpretation:
The formation of the observed product C is to be explained.
Concept introduction:
Pericyclic reactions deal with electrons shift in a concerted manner. Moreover, in these reactions the breaking of reactant bonds and the formation of product bonds are formed at the same time.i.e., without the formation of intermediates. The selection rules of pericyclic reaction deal mostly on the rates of reaction and does not tell anything about position of equilibrium and equilibrium constant values.
Trending nowThis is a popular solution!

Chapter 28 Solutions
Organic Chemistry
- 10. The most important reason why Br- is a better nucleophile than Cl-is ___. A. polarizability; B. size; C. solvation; D. basicity; E. polarity. Please include all steps. Thanks!arrow_forwardPredicting the qualitative acid-base properties of salts Consider the following data on some weak acids and weak bases: base acid Ка K₁₁ name formula name formula nitrous acid HNO2 4.5×10 4 pyridine CHEN 1.7 × 10 9 4 hydrofluoric acid HF 6.8 × 10 methylamine CH3NH2 | 4.4 × 10¯ Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. solution 0.1 M NaNO2 0.1 M KF pH choose one v choose one v 0.1 M C5H5NHBr 0.1 M CH3NH3CI choose one v ✓ choose one 1 (lowest) 2 ☑ 3 4 (highest) 000 18 Ararrow_forward4. The major product from treatment of 2-propanol with the Jonesreagent is ___.A. acetone; B. none of the other answers is correct C. propene; D.propanoic acid; E carbon dioxide. Please include all steps! Thank you!arrow_forward
- 7. All of the following compounds that are at the same oxidation levelare ___.u. methyl epoxide, v. propyne, w. propanal, x. propene,y. 2,2-dihydroxypropane, z. isopropanol?A. u,v,w,y; B. u,v,w; C. v,w,y,z; D. v, z; E. x,y,z Please include all steps. Thank you!arrow_forward9. Which one of the following substituents is the worst leaving group inan SN2 reaction? A. -NH2; B. -OH; C. –F; D. NH3; E. H2O Please include all steps. Thanks!arrow_forwardUsing the general properties of equilibrium constants At a certain temperature, the equilibrium constant K for the following reaction is 2.5 × 105: CO(g) + H2O(g) CO2(g) + H2(g) Use this information to complete the following table. Suppose a 7.0 L reaction vessel is filled with 1.7 mol of CO and 1.7 mol of H2O. What can you say about the composition of the mixture in the vessel at equilibrium? What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. CO2(9)+H2(g) CO(g)+H₂O(g) What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 3 CO(g)+3H2O(g) = 3 CO2(g)+3H2(g) There will be very little CO and H2O. x10 There will be very little CO2 and H2. 000 Neither of the above is true. K = ☐ K = ☐ 18 Ararrow_forward
- 8. When ethane thiol is treated with hydrogen peroxide the product is___.A. ethane disulfide; B. diethyl sulfide; C. ethane sulfoxide; D. ethanesulfate; E. ethyl mercaptan. Please include all steps. Thanks!arrow_forward5. The major product of the three step reaction that takes place when 1-propanol is treated with strong acid is?A. dipropyl ether; B. propene; C. propanal; D. isopropyl propyl ether;E. 1-hexanol Please include all steps. Thank you!arrow_forward6. The formula of the product of the addition of HCN to benzaldehydeis ___.A. C8H7NO; B. C8H6NO; C. C14H11NO; D. C9H9NO; E. C9H8NO Please include all steps. Thank you!arrow_forward
- Predicting the qualitative acid-base properties of salts Consider the following data on some weak acids and weak bases: base acid K K a name formula name formula nitrous acid HNO2 4.5×10 hydroxylamine HONH2 1.1 × 10 8 hypochlorous acid HCIO 8 3.0 × 10 methylamine CH3NH2 | 4.4 × 10¯ 4 Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. 0.1 M KCIO solution PH choose one 0.1 M NaNO2 0.1 M CH3NH3Br 0.1 M NaBr choose one ✓ choose one v ✓ choose one 1 (lowest) ☑ 2 3 4 (highest)arrow_forwardFor this Orgo problem, don't worry about question 3 below it. Please explain your thought process, all your steps, and also include how you would tackle a similar problem. Thank you!arrow_forwardUsing the general properties of equilibrium constants At a certain temperature, the equilibrium constant K for the following reaction is 0.84: H2(g) + 2(g) 2 HI(g) = Use this information to complete the following table. Suppose a 34. L reaction vessel is filled with 0.79 mol of HI. What can you say about the composition of the mixture in the vessel at equilibrium? There will be very little H2 and 12. ☐ x10 There will be very little HI. Neither of the above is true. What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 2 HI(g) H₂(9)+12(9) K = What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 2 H2(g)+212(9) 4 HI(g) K = ☐ ☑arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





