
Concept explainers
(a)
Interpretation:
In conjugated triene,
Concept introduction:
The molecular orbital is a combination of two atomic orbitals. It is used to represent the regions in a molecule where the electron is likely to be present in an orbital. It represents the wave-like nature of an electron in a molecule. It may be symmetric or antisymmetric. It may be bonding, antibonding or non-bonding. It may be HOMO or LUMO.
Answer to Problem 28.2P
In
Explanation of Solution
The structure of
The electron configuration of carbon atom is
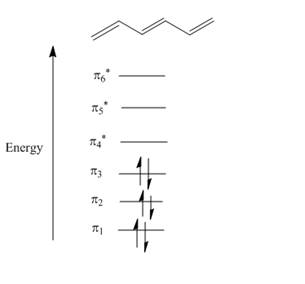
Figure 1
The atomic p orbital of carbon atom overlapped together to form molecular orbital. A total of six molecular orbitals are formed due to six carbon atomic orbital overlapping in
(b)
Interpretation:
Each molecular orbital is to be classified as symmetric and antisymmetric.
Concept introduction:
The molecular orbital is a combination of two atomic orbitals. It is used to represent the regions in a molecule where the electron is likely to be present in an orbital. It represents the wave-like nature of an electron in a molecule. It may be symmetric or antisymmetric. It may be bonding, antibonding or non-bonding. It may be HOMO or LUMO.
Answer to Problem 28.2P
The molecular orbitals
Explanation of Solution
In molecular orbital theory, the MO is said to be symmetric or anti-symmetric is depend on the relative phase of the two terminal carbons. In symmetric MO, the peaks reflect across the reference plane into the peaks and troughs reflect into troughs. On the other hand, in antisymmetric MO, the peaks reflect into troughs and vice versa. According to the general principle, the even number molecular orbitals are antisymmetric and odd number molecular orbitals are symmetric. Therefore, the molecular orbitals
The odd number of molecular orbitals is symmetric molecular orbitals that are
(c)
Interpretation:
Bonding and antibonding molecular orbitals are to be identified.
Concept introduction:
The molecular orbital is a combination of two atomic orbitals. It is used to represent the regions in a molecule where the electron is likely to be present in an orbital. It represents the wave-like nature of an electron in a molecule. It may be symmetric or antisymmetric. It may be bonding, antibonding or non-bonding. It may be HOMO or LUMO.
Answer to Problem 28.2P
The molecular orbital
Explanation of Solution
In
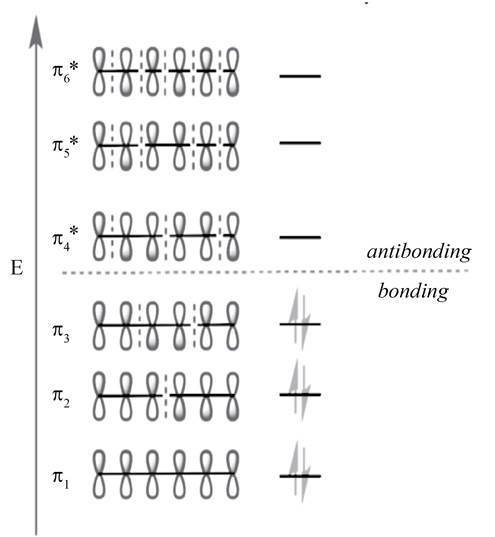
Figure 2
The molecular orbital with lower energy are bonding MO
(d)
Interpretation:
Among the molecular orbitals, the frontier molecular orbital is to be identified.
Concept introduction:
The molecular orbital is a combination of two atomic orbitals. It is used to represent the regions in a molecule where the electron is likely to be present in an orbital. It represents the wave-like nature of an electron in a molecule. It may be symmetric or antisymmetric. It may be bonding, antibonding or non-bonding. It may be HOMO or LUMO.
Answer to Problem 28.2P
The molecular orbital
Explanation of Solution
The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) is known as frontier molecular orbitals. In
The molecular orbital
(e)
Interpretation:
Whether the phase at terminal carbons in HOMO orbitals is the same or different is to be stated.
Concept introduction:
The molecular orbital is a combination of two atomic orbitals. It is used to represent the regions in a molecule where the electron is likely to be present in an orbital. It represents the wave-like nature of an electron in a molecule. It may be symmetric or antisymmetric. It may be bonding, antibonding or non-bonding. It may be HOMO or LUMO.
Answer to Problem 28.2P
The HOMO orbital that is
Explanation of Solution
According to the general principle, the even number molecular orbitals are antisymmetric or its phase terminal carbons are different and odd number molecular orbitals are symmetric or its phase terminal carbons are same. The HOMO orbital is
The HOMO orbital is
(f)
Interpretation:
Whether the phase at terminal carbons in LUMO orbitals is the same or different is to be stated.
Concept introduction:
The molecular orbital is a combination of two atomic orbitals. It is used to represent the regions in a molecule where the electron is likely to be present in an orbital. It represents the wave-like nature of an electron in a molecule. It may be symmetric or antisymmetric. It may be bonding, antibonding or non-bonding. It may be HOMO or LUMO.
Answer to Problem 28.2P
The LUMO orbital that is
Explanation of Solution
According to the general principle, the even number molecular orbitals are antisymmetric or its phase terminal carbons are different and odd number molecular orbitals are symmetric or its phase terminal carbons are same. The LUMO orbital is
The LUMO orbital is
Want to see more full solutions like this?
Chapter 28 Solutions
Organic Chemistry
- 1. For each of the reaction "railroads" below, you are either asked to give the structure(s) of the starting material(s) or product(s), or provide reagents/conditions to accomplish the transformation, as indicated by the boxes. a. NaOMe H+ .CO,H HO₂C MeOH (excess) MeOH H3C Br يع CH3 1. LiAlH4 2. H3O+ 3. PBг3 H3C 1. Et-Li 2. H3O+ -CO₂Me -CO₂Me OH CH3 CH3 ল CH3arrow_forwardPredict the intermediate 1 and final product 2 of this organic reaction: NaOMe ག1, ད།་, - + H You can draw 1 and 2 in any arrangement you like. 2 work up Note: if either 1 or 2 consists of a pair of enantiomers, just draw one structure using line bonds instead of 3D (dash and wedge) bonds at the chiral center. Explanation Check Click and drag to start drawing a structure. Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Parrow_forwardWhat is the total energy cost associated with the compound below adopting the shown conformation? CH3 HH DH CH3arrow_forward
- ΗΝ, Draw Final Product C cyclohexanone pH 4-5 Edit Enamine H3O+ CH3CH2Br THF, reflux H Edit Iminium Ionarrow_forwardHow many hydrogen atoms are connected to the indicated carbon atom?arrow_forwardIdentify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forward
- Identify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forwardRank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forward
- Rank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forwardWhy isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

