
(a)
Interpretation:
Each of the following reactions involves a sequence of two pericyclic reactions. The intermediate X involved in each reaction, and pericyclic reactions involved are to be depicted.
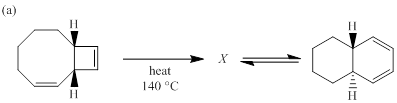
Concept introduction:
Electrocyclic reactions are a pericyclic reaction which occur intramolecularly. These reactions will result in the formation of ring compounds under the influence of heat or light. Notably, in this process one new sigma bond is formed and one old π-bond is consumed. Intriguingly, the reverse ring opening electrocyclic reaction can also be possible to occur under the same reaction mechanism but in reverse manner. In phase orbital overlap results in symmetry allowed electrocyclic reactions. Selection rules of electrocyclic reactions are;
| No. of electrons | Activation mode | Stereochemistry of rotation |
| 4n | Thermal Photochemical |
Con Dis |
| 4n + 2 | Thermal Photochemical |
Dis Con |
(b)
Interpretation:
Each of the following reactions involves a sequence of two pericyclic reactions. The intermediate Y involved in each reaction, and pericyclic reactions involved are to be depicted.
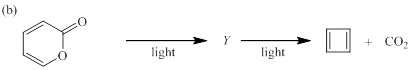
Concept introduction:
Electrocyclic reactions are a pericyclic reaction which occur intramolecularly. These reactions will result in the formation of ring compounds under the influence of heat or light. Notably, in this process one new sigma bond is formed and one old π-bond is consumed. Intriguingly, the reverse ring opening electrocyclic reaction can also be possible to occur under the same reaction mechanism but in reverse manner.
Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
Organic Chemistry Study Guide and Solutions
- Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forwardCan I please get help with this?arrow_forward
- Can I please get help with identifying these?arrow_forward4. Calculate the pH of a 0.10 M acetic acid (CH3COOH) solution if the Ka of acetic acid = 1.8 x 10-5arrow_forwardDraw the Zaitsev product of the dehydration of this alcohol. + I X 5 OH ざ~ TSOH Click and drag to start drawing a structure.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

