
Concept explainers
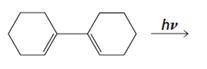
Draw the product of each of the following reactions:
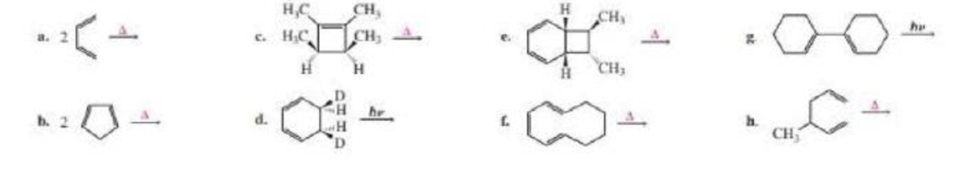
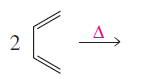
(a)
Interpretation:
The product formed in the given reaction has to be drawn.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Pericyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for pericyclic reactions are listed below
A photochemical reaction takes place when a reactant absorbs light and a thermal reaction takes place without the absorption of light.
Woodward – Hoffmann rules for the configuration of pericyclic reactions are,
Answer to Problem 23P
Product formed in the given reaction is,
Explanation of Solution
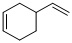
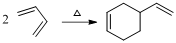
Given reaction is,
Herein, the compound has an even number of
Therefore the structure of the product formed in the reaction is,
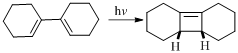
(b)
Interpretation:
The product formed in the given reaction has to be drawn.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Pericyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for Pericyclic reactions are listed below
A photochemical reaction takes place when a reactant absorbs light and a thermal reaction takes place without the absorption of light.
Woodward – Hoffmann rules for the configuration of pericyclic reactions are,
Answer to Problem 23P
Product of given reaction is,
Explanation of Solution
Given reaction is,
Herein, the compound has an even number of
Therefore the structure of the product formed in the reaction is,
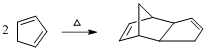
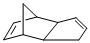
(c)
Interpretation:
The product formed in the given reaction has to be drawn.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Pericyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for Pericyclic reactions are listed below
A photochemical reaction takes place when a reactant absorbs light and a thermal reaction takes place without the absorption of light.
Woodward – Hoffmann rules for the configuration of pericyclic reactions are,
Answer to Problem 23P
The product of the given reaction is,
Explanation of Solution
Given reaction is,
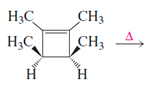
Here in the compound in which the four methyl substituent point in opposite directions, then according to Woodward-Hoffmann rules, they will be “cis” in the ring-closed product when ring closure is conrotatory and “trans” in the ring-closed product when ring closure is disrotatory.
Therefore the structure of the product formed in the reaction is,
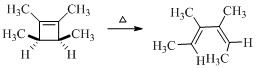
(d)
Interpretation:
The product formed in the given reaction has to be drawn.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Pericyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for Pericyclic reactions are listed below
A photochemical reaction takes place when a reactant absorbs light and a thermal reaction takes place without the absorption of light.
Woodward – Hoffmann rules for the configuration of pericyclic reactions are,
Answer to Problem 23P
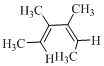
Product of the given reaction is,
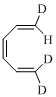
Explanation of Solution
Given reaction is,
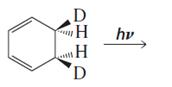
Here in the compound in which the two D and H substituent point in same directions, then according to Woodward-Hoffmann rules, they will be “trans” in the ring-closed product when ring closure is disrotatory and cis in the ring-closed product when ring closure is conrotatory.
Therefore the structure of the product formed in the reaction is,
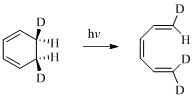
(e)
Interpretation:
The product formed in the given reaction has to be drawn.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Pericyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for Pericyclic reactions are listed below
A photochemical reaction takes place when a reactant absorbs light and a thermal reaction takes place without the absorption of light.
Woodward – Hoffmann rules for the configuration of pericyclic reactions are,
Answer to Problem 23P
The product of given reaction is,
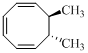
Explanation of Solution
Given reaction is,
Here in the compound have two
Therefore the structure of the product formed in the reaction is,
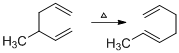
(f)
Interpretation:
The product formed in the given reaction has to be drawn.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Pericyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for Pericyclic reactions are listed below
A photochemical reaction takes place when a reactant absorbs light and a thermal reaction takes place without the absorption of light.
Woodward – Hoffmann rules for the configuration of pericyclic reactions are,
Answer to Problem 23P
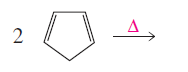
Product of given reaction is,
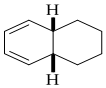
Explanation of Solution
Given reaction is,
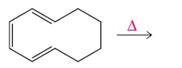
The reaction carried out under thermal conditions then according with the several Woodward - Hoffmann rules the probable structure formed in the given reaction is,
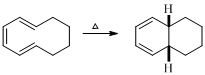
(g)
Interpretation:
The product formed in the given reaction has to be drawn.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Pericyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for Pericyclic reactions are listed below
A photochemical reaction takes place when a reactant absorbs light and a thermal reaction takes place without the absorption of light.
Woodward – Hoffmann rules for the configuration of pericyclic reactions are,
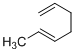
Answer to Problem 23P
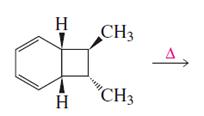
The product of given reaction is,
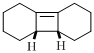
Explanation of Solution
Given reaction is,
Here in the compound have two
(h)
Interpretation:
The product formed in the given reaction has to be drawn.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Pericyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for Pericyclic reactions are listed below
A photochemical reaction takes place when a reactant absorbs light and a thermal reaction takes place without the absorption of light.
Woodward – Hoffmann rules for the configuration of pericyclic reactions are,
Answer to Problem 23P
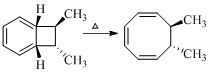
Product of given reaction is,
Explanation of Solution
Given reaction is,
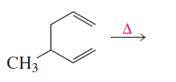
According to the Woodward-Hoffmann rules, the reactant coverts to product under thermal condition and the number of
The structure of the product formed in the reaction is,
Want to see more full solutions like this?
Chapter 28 Solutions
Organic Chemistry (8th Edition)
- true or false, given that a 20.00 mL sample of NaOH took 24.15 mL of 0.141 M HCI to reach the endpoint in a titration, the concentration of the NaOH is 1.17 M.arrow_forwardin the bromothymol blue experiment, pKa was measured. A closely related compound has a Ka of 2.10 x 10-5. What is the pKa?a) 7.1b) 4.7c) 2.0arrow_forwardcalculate the equilibrium concentration of H2 given that K= 0.017 at a constant temperature for this reaction. The inital concentration of HBr is 0.050 M.2HBr(g) ↔ H2(g) + Br2(g)a) 4.48 x 10-2 M b) 5.17 x 10-3 Mc) 1.03 x 10-2 Md) 1.70 x 10-2 Marrow_forward
- true or falsegiven these two equilibria with their equilibrium constants:H2(g) + CI2(l) ↔ 2HCI(g) K= 0.006 CI2(l) ↔ CI2(g) K= 0.30The equilibrium contstant for the following reaction is 1.8H2(g) + CI2 ↔ 2HCI(g)arrow_forwardI2(g) + CI2(g) ↔ 2ICIK for this reaction is 81.9. Find the equilibrium concentration of I2 if the inital concentration of I2 and CI2 are 0.010 Marrow_forwardtrue or false,the equilibrium constant for this reaction is 0.50.PCI5(g) ↔ PCI3(g) + CI2(g)Based on the above, the equilibrium constant for the following reaction is 0.25.2PCI5(g) ↔. 2PCI3(g) + 2CI2(g)arrow_forward
- true or false, using the following equilibrium, if carbon dioxide is added the equilibrium will shift toward the productsC(s) + CO2(g) ↔ 2CO(g)arrow_forward2S2O2/3- (aq) + I2 (aq) ---> S4O2/6- (aq) +2I- (aq) Experiment I2 (M) S2O3- (M) Initital Rate (M/s) 1 0.01 0.01 0.0004 2 0.01 0.02 0.0004 3 0.02 0.01 0.0008 Calculate the overall order for this reaction using the table data a) 3b) 0c) 2d) 1arrow_forwardthe decomposition of N2O5 is the first order with a half-life of 1.98 minutes. If the inital concentration of N2O5 is 0.200 M, what is the concentration after 6 minutes?a) 0.612 Mb) 0.035 Mc) 0.024 Md) 0.100 Marrow_forward
- 20.00 mL of 0.150 M HCI is titrated with 0.075 M NaOH. What volume of NaOH is needed?a) 50 mLb) 20 mLc) 40 mLd) 26.66 mLarrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCI. What is the molarity of the HCI?a) 0.150 Mb) 0.079 Mc) 0.025 Md) 0.050 Marrow_forwardin the following reaction, the OH- acts as which of these?NO2- (aq) + H2O (l) ⇌ OH- (aq) + HNO2 (aq)a) not a weak acidb) basec) acidarrow_forward
