
CONNECT FOR THERMODYNAMICS: AN ENGINEERI
9th Edition
ISBN: 9781260048636
Author: CENGEL
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2.8, Problem 107P
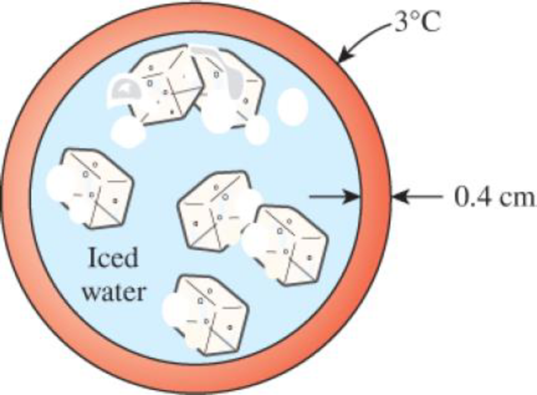
A hollow spherical iron container whose outer diameter is 40 cm and thickness is 0.4 cm is filled with iced water at 0°C. If the outer surface temperature is 3°C, determine the approximate rate of heat loss from the sphere, and the rate at which ice melts in the container.
FIGURE P2–107
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
PLEASE SOLVE STEP BY STEP WITHOUT ARTIFICIAL INTELLIGENCE OR CHATGPT
SOLVE BY HAND STEP BY STEP
PLEASE SOLVE STEP BY STEP WITHOUT ARTIFICIAL INTELLIGENCE OR CHATGPT
SOLVE BY HAND STEP BY STEP
PLEASE SOLVE STEP BY STEP WITHOUT ARTIFICIAL INTELLIGENCE OR CHATGPT
SOLVE BY HAND STEP BY STEP
Chapter 2 Solutions
CONNECT FOR THERMODYNAMICS: AN ENGINEERI
Ch. 2.8 - What is the difference between the macroscopic and...Ch. 2.8 - What is total energy? Identify the different forms...Ch. 2.8 - List the forms of energy that contribute to the...Ch. 2.8 - How are heat, internal energy, and thermal energy...Ch. 2.8 - What is mechanical energy? How does it differ from...Ch. 2.8 - Portable electric heaters are commonly used to...Ch. 2.8 - Natural gas, which is mostly methane CH4, is a...Ch. 2.8 - Consider the falling of a rock off a cliff into...Ch. 2.8 - Electric power is to be generated by installing a...Ch. 2.8 - The specific kinetic energy of a moving mass is...
Ch. 2.8 - Determine the specific kinetic energy of a mass...Ch. 2.8 - Calculate the total potential energy, in Btu, of...Ch. 2.8 - Determine the specific potential energy, in kJ/kg,...Ch. 2.8 - An object whose mass is 100 kg is located 20 m...Ch. 2.8 - A water jet that leaves a nozzle at 60 m/s at a...Ch. 2.8 - Consider a river flowing toward a lake at an...Ch. 2.8 - At a certain location, wind is blowing steadily at...Ch. 2.8 - What is the caloric theory? When and why was it...Ch. 2.8 - In what forms can energy cross the boundaries of a...Ch. 2.8 - What is an adiabatic process? What is an adiabatic...Ch. 2.8 - When is the energy crossing the boundaries of a...Ch. 2.8 - Consider an automobile traveling at a constant...Ch. 2.8 - A room is heated by an iron that is left plugged...Ch. 2.8 - A room is heated as a result of solar radiation...Ch. 2.8 - A gas in a pistoncylinder device is compressed,...Ch. 2.8 - A small electrical motor produces 5 W of...Ch. 2.8 - A car is accelerated from rest to 85 km/h in 10 s....Ch. 2.8 - A construction crane lifts a prestressed concrete...Ch. 2.8 - Determine the torque applied to the shaft of a car...Ch. 2.8 - A spring whose spring constant is 200 lbf/in has...Ch. 2.8 - How much work, in kJ, can a spring whose spring...Ch. 2.8 - A ski lift has a one-way length of 1 km and a...Ch. 2.8 - The engine of a 1500-kg automobile has a power...Ch. 2.8 - A damaged 1200-kg car is being towed by a truck....Ch. 2.8 - As a spherical ammonia vapor bubble rises in...Ch. 2.8 - A steel rod of 0.5 cm diameter and 10 m length is...Ch. 2.8 - What are the different mechanisms for transferring...Ch. 2.8 - For a cycle, is the net work necessarily zero? For...Ch. 2.8 - On a hot summer day, a student turns his fan on...Ch. 2.8 - Water is being heated in a closed pan on top of a...Ch. 2.8 - An adiabatic closed system is accelerated from 0...Ch. 2.8 - A fan is to accelerate quiescent air to a velocity...Ch. 2.8 - A vertical pistoncylinder device contains water...Ch. 2.8 - At winter design conditions, a house is projected...Ch. 2.8 - A water pump increases the water pressure from 15...Ch. 2.8 - The lighting needs of a storage room are being met...Ch. 2.8 - A university campus has 200 classrooms and 400...Ch. 2.8 - Consider a room that is initially at the outdoor...Ch. 2.8 - An escalator in a shopping center is designed to...Ch. 2.8 - Consider a 2100-kg car cruising at constant speed...Ch. 2.8 - Prob. 51PCh. 2.8 - What is mechanical efficiency? What does a...Ch. 2.8 - How is the combined pumpmotor efficiency of a pump...Ch. 2.8 - Can the combined turbinegenerator efficiency be...Ch. 2.8 - Consider a 2.4-kW hooded electric open burner in...Ch. 2.8 - The steam requirements of a manufacturing facility...Ch. 2.8 - Reconsider Prob. 256E. Using appropriate software,...Ch. 2.8 - A 75-hp (shaft output) motor that has an...Ch. 2.8 - Prob. 59PCh. 2.8 - An exercise room has six weight-lifting machines...Ch. 2.8 - A room is cooled by circulating chilled water...Ch. 2.8 - The water in a large lake is to be used to...Ch. 2.8 - A 7-hp (shaft) pump is used to raise water to an...Ch. 2.8 - A geothermal pump is used to pump brine whose...Ch. 2.8 - At a certain location, wind is blowing steadily at...Ch. 2.8 - Reconsider Prob. 265. Using appropriate software,...Ch. 2.8 - Water is pumped from a lower reservoir to a higher...Ch. 2.8 - An 80-percent-efficient pump with a power input of...Ch. 2.8 - Water is pumped from a lake to a storage tank 15 m...Ch. 2.8 - Large wind turbines with a power capacity of 8 MW...Ch. 2.8 - A hydraulic turbine has 85 m of elevation...Ch. 2.8 - The water behind Hoover Dam in Nevada is 206 m...Ch. 2.8 - An oil pump is drawing 44 kW of electric power...Ch. 2.8 - A wind turbine is rotating at 15 rpm under steady...Ch. 2.8 - How does energy conversion affect the environment?...Ch. 2.8 - What is acid rain? Why is it called a rain? How do...Ch. 2.8 - Why is carbon monoxide a dangerous air pollutant?...Ch. 2.8 - What is the greenhouse effect? How does the excess...Ch. 2.8 - What is smog? What does it consist of? How does...Ch. 2.8 - Consider a household that uses 14,000 kWh of...Ch. 2.8 - When a hydrocarbon fuel is burned, almost all of...Ch. 2.8 - Prob. 82PCh. 2.8 - A typical car driven 20,000 km a year emits to the...Ch. 2.8 - Prob. 84PCh. 2.8 - What are the mechanisms of heat transfer?Ch. 2.8 - Which is a better heat conductor, diamond or...Ch. 2.8 - How does forced convection differ from natural...Ch. 2.8 - What is a blackbody? How do real bodies differ...Ch. 2.8 - Define emissivity and absorptivity. What is...Ch. 2.8 - Does any of the energy of the sun reach the earth...Ch. 2.8 - The inner and outer surfaces of a 5-m 6-m brick...Ch. 2.8 - The inner and outer surfaces of a 0.5-cm-thick 2-m...Ch. 2.8 - Reconsider Prob. 292. Using appropriate software,...Ch. 2.8 - Prob. 94PCh. 2.8 - Prob. 95PCh. 2.8 - Prob. 96PCh. 2.8 - Prob. 97PCh. 2.8 - For heat transfer purposes, a standing man can be...Ch. 2.8 - Prob. 99PCh. 2.8 - Prob. 100PCh. 2.8 - A 1000-W iron is left on the ironing board with...Ch. 2.8 - A 7-cm-external-diameter, 18-m-long hot-water pipe...Ch. 2.8 - A thin metal plate is insulated on the back and...Ch. 2.8 - Reconsider Prob. 2103. Using appropriate software,...Ch. 2.8 - The outer surface of a spacecraft in space has an...Ch. 2.8 - Prob. 106PCh. 2.8 - A hollow spherical iron container whose outer...Ch. 2.8 - Some engineers have developed a device that...Ch. 2.8 - Consider a classroom for 55 students and one...Ch. 2.8 - Consider a homeowner who is replacing his...Ch. 2.8 - Prob. 111RPCh. 2.8 - The U.S. Department of Energy estimates that...Ch. 2.8 - A typical household pays about 1200 a year on...Ch. 2.8 - Prob. 114RPCh. 2.8 - Prob. 115RPCh. 2.8 - Prob. 116RPCh. 2.8 - Consider a TV set that consumes 120 W of electric...Ch. 2.8 - Water is pumped from a 200-ft-deep well into a...Ch. 2.8 - Consider a vertical elevator whose cabin has a...Ch. 2.8 - Prob. 120RPCh. 2.8 - In a hydroelectric power plant, 65 m3/s of water...Ch. 2.8 - The demand for electric power is usually much...Ch. 2.8 - The pump of a water distribution system is powered...Ch. 2.8 - Prob. 124RPCh. 2.8 - A 2-kW electric resistance heater in a room is...Ch. 2.8 - Prob. 126FEPCh. 2.8 - A 75-hp compressor in a facility that operates at...Ch. 2.8 - On a hot summer day, the air in a well-sealed room...Ch. 2.8 - A fan is to accelerate quiescent air to a velocity...Ch. 2.8 - A 900-kg car cruising at a constant speed of 60...Ch. 2.8 - Prob. 131FEPCh. 2.8 - Prob. 132FEPCh. 2.8 - A 2-kW pump is used to pump kerosene ( = 0.820...Ch. 2.8 - Prob. 134FEPCh. 2.8 - Prob. 135FEPCh. 2.8 - Prob. 136FEPCh. 2.8 - Prob. 137FEPCh. 2.8 - Heat is transferred steadily through a...Ch. 2.8 - The roof of an electrically heated house is 7 m...
Additional Engineering Textbook Solutions
Find more solutions based on key concepts
Using your text editor, enter (that is, type in) the C++ program shown in Display 1.8. Be certain to type the f...
Problem Solving with C++ (10th Edition)
A nozzle at A discharges water with an initial velocity of 36 ft/s at an angle with the horizontal. Determine ...
Vector Mechanics For Engineers
The solid steel shaft AC has a diameter of 25 mm and is supported by smooth bearings at D and E. It is coupled ...
Mechanics of Materials (10th Edition)
Write a summary list of the problem-solving steps identified in the chapter, using your own words.
BASIC BIOMECHANICS
In Exercises 1 through 22, determine the output displayed in the text box or list box by the lines of code.
Introduction To Programming Using Visual Basic (11th Edition)
What types of coolant are used in vehicles?
Automotive Technology: Principles, Diagnosis, And Service (6th Edition) (halderman Automotive Series)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Consider the bar, shown in Figure 1 that undergoes axial displacement due to both a distributed load and a point force. The bar is of cross-sectional area A = 1.10-3 m², and has a modulus of elasticity E = 100 GPa. 1(x) = 5 kN/m x=0.0 x=2.0 2.0m 10 kN Figure 1: Bar domain with varying distributed forces. a) The general form of the governing equations describing the bar's displacement, u(x), is given by, d (AE du(x)) -) +1(x) = 0. d.x dx What are the accompanying boundary conditions for this bar? b) Using the mesh in Figure 2, form the basis functions associated with element 2 and write the FEM approximation over the element. 1 2 3 1 2 1m 1m Figure 2: Mesh of 2 elements. Elements are numbered with underlines. c) The general form of the element stiffness matrix system, with nodes indexed by i and j, is, AE Uj N;(x)l(x)dx – Ng(0)f(0) ¥ [4]}]{{}}={{{}\(\\+} + {N(2)f(2) = N (0)5() }, (1) 0, respectively. L = (2) where f(2) and f(0) denote the boundary forces at positions x 2 and x Evaluate…arrow_forwardanswer pleasearrow_forwardamination) Question 1 Consider the bar, shown in Figure 1, that undergoes axial displacement due to both a distributed load and a point force. The bar is of cross-sectional area A = 1.103 m2, and has a modulus of elasticity E = 100 GPa. 1(x) = 5 kN/m 10 kN X x=0.0 x=2.0 2.0m Figure 1: Bar domain with varying distributed forces. a) The general form of the governing equations describing the bar's displacement, u(x), is given by, d (AE du(x)) + 1(x) = 0. dx dx What are the accompanying boundary conditions for this bar? MacBook Air a 会 DII F5 F6 F7 F8 80 F3 F4 0/ 20 [8 marksl 8 FOarrow_forward
- show workingarrow_forwardCFD help Figure 3: Advection equation, solution for three different timesteps. Q1) Provide an explanation what conditions and numerical setup could explain the curves. Identify which of the three curves is the first, second and third timestep.arrow_forwardanswer pleasearrow_forward
- Figure 3 shows the numerical solution of the advection equation for a scalar u along x at three consecutive timesteps. 1.0 0.8- 0.6 0.4- 0.2 0.0 00 -0.2 -0.4 -0.6- 3.0 3.5 4.0 4.5 5.0 5.5 6.0 6.5 Figure 3: Advection equation, solution for three different timesteps.arrow_forwardQuestion 2 Figure 3 shows the numerical solution of the advection equation for a scalar u along x at three consecutive timesteps. 1.0 0.8- 0.6- 0.4- 0.2- 0.0- -0.2- -0.4- -0.6 3.0 3.5 4.0 4.5 5.0 5.5 6.0 6.5 Figure 3: Advection equation, solution for three different timesteps. a) Provide an explanation what conditions and numerical setup could explain the curves. Identify which of the three curves is the first, second and third timestep. b) Consider explicit schemes with central and upwind discretisations. Explain how each of these candidate discretisations could produce the behaviour shown in Figure 3. c) Determine the CFL number that was used in the simulation for each of the candidate schemes for all possible updates. Assume that the timestep and mesh-width used are constant. Read the data to two digits of accuracy from Figure 4 shown at the end of the question, which is an enlarged version of Figure 3. Demonstrate your method and input data for one calculation, but then use a…arrow_forwardanswer pleasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=NyOYW07-L5g;License: Standard youtube license