
(a)
Interpretation:
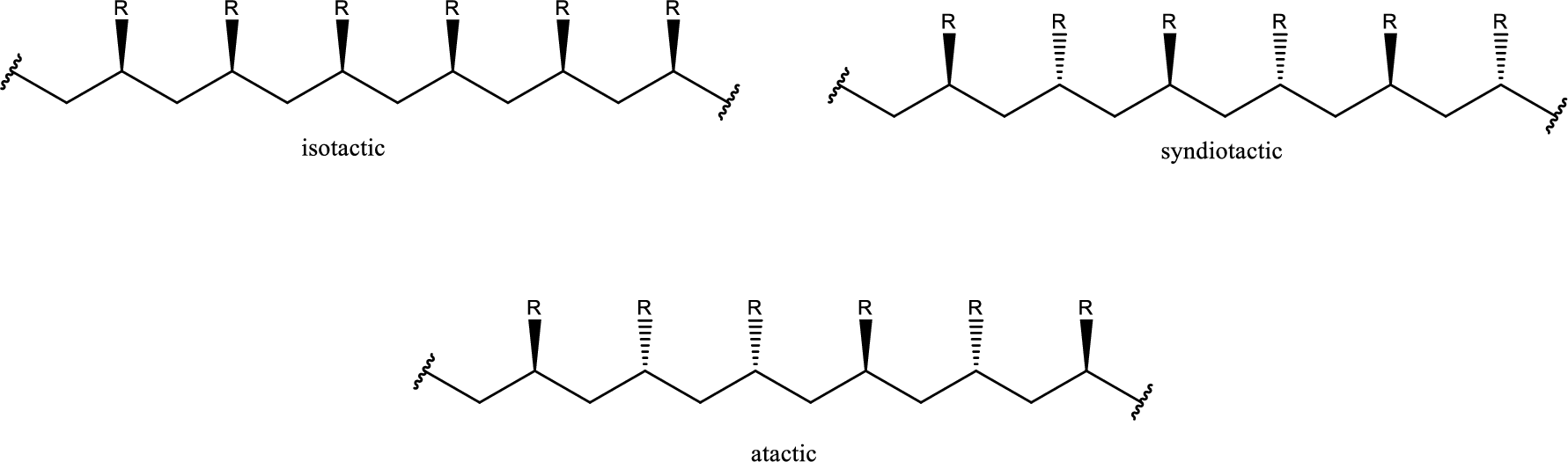
Syndiotactic poly (vinyl chloride) segments need to be drawn.
Concept introduction:
If the polymer formed has chiral centers then with the relative configuration of the chiral centers the
(b)
Interpretation:
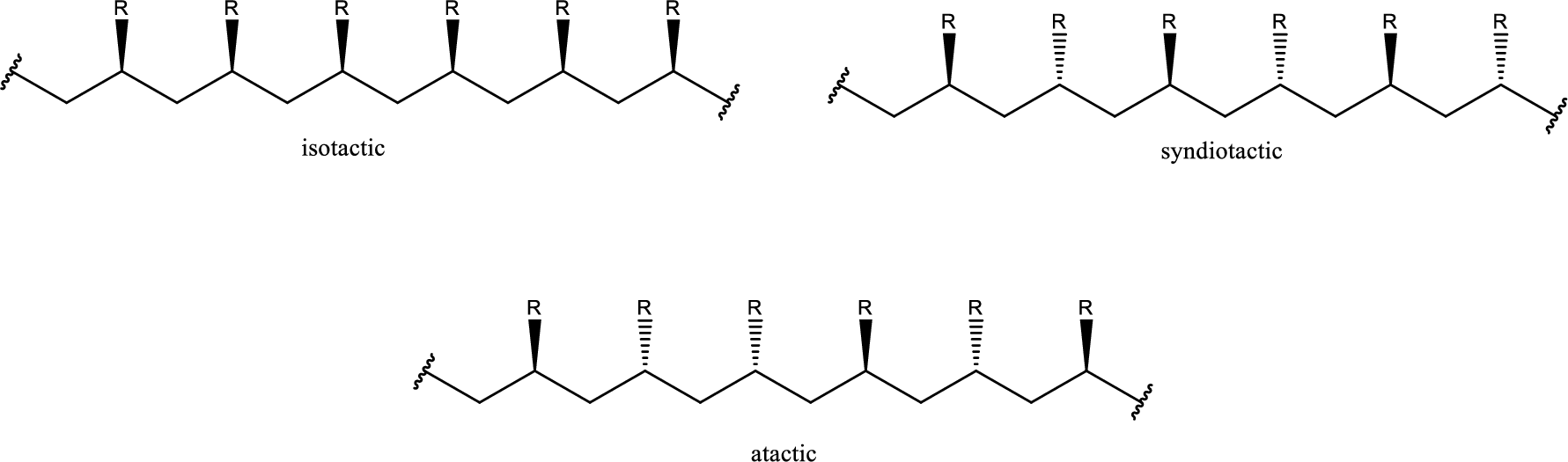
Isotactic polystyrene segments need to be drawn.
Concept introduction:
If the polymer formed has chiral centers then with the relative configuration of the chiral centers the polymers can be classified as isotactic, Syndiotactic or atactic. When monosubstituted ethylenes are used as monomer the polymer formed has huge number of chiral centers. Isotactic means all the chiral center has the same configuration. Syndiotactic means the chiral centers has alternating configuration. Atactic means the chiral centers are in random arrangement.
Want to see the full answer?
Check out a sample textbook solution
Chapter 27 Solutions
Organic Chemistry Third Edition + Electronic Solutions Manual And Study Guide
- Predict the major organic product(s), if any, of the following reactions. Assume all reagents are in excess unless otherwise indicated.arrow_forwardHow many signals would you expect to find in the 1 H NMR spectrum of each given compound? Part 1 of 2 2 Part 2 of 2 HO 5 ☑ Х IIIIII***** §arrow_forwardA carbonyl compound has a molecular ion with a m/z of 86. The mass spectra of this compound also has a base peak with a m/z of 57. Draw the correct structure of this molecule. Drawingarrow_forward
- Can you draw this using Lewis dot structures and full structures in the same way they are so that I can better visualize them and then determine resonance?arrow_forwardSynthesize the following compound from cyclohexanol, ethanol, and any other needed reagentsarrow_forwardFor a titration of 20.00 mL of 0.0500 M H2SO4 with 0.100 M KOH, calculate the pH at each of the following volume of KOH used in the titration: 1) before the titration begin; 2) 10.00 mL; 3) 20.00 mL; 4) 30.00 mL. Ka2 = 1.20×10-2 for H2SO4.arrow_forward
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s) Be sure to account for all bond-breaking and bond-making steps Problem 73 of 10 Drawing Amows ro HO Donearrow_forward12. Synthesize the following target molecules (TMs) using the specified starting materials. .CI a) HO3S SM TM b) HO- SMarrow_forwardFor a titration of 20.00 mL of 0.0500 M H2SO4 with 0.100 M KOH, calculate the pH at each of the following volume of KOH used in the titration: 1) before the titration begin; 2) 10.00 mL; 3) 20.00 mL; 4) 30.00 mL. Ka2 = 1.20×10-2 for H2SO4.arrow_forward
- Write the systematic name of each organic molecule: structure name show work. don't give Ai generated solutionarrow_forwardShow work with explanation needed. Don't give Ai generated solutionarrow_forwardA Elschboard Part of SpeechT-D Alt Leaming App app.aktiv.com Curved arrows are used to illustrate the flow of electrons. Using the provided resonance structures, draw the curved electron- pushing arrows to show the interconversion between resonance hybrid contributors. Be sure to account for all bond-breaking and bond-making steps. Include all lone pairs and formal charges in the structures. Problem 45 of 10 I Select to Add Arrows N Please selarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





