
Concept explainers
Oligonucleotide Synthesis
In Section
available for use as primers for PCR and as probes for cloning DNA. Here we will examine how these oligonucleotides are prepared.
The method bears many similarities to the Merrifield solid-phase synthesis of peptides. A starter unit is attached to a solid support, nucleosides are attached one-by-one until the sequence is complete, whereupon the target oligonucleotide is removed from the support and purified. Like solid-phase peptide synthesis, the preparation of oligonucleotides relies heavily on protecting groups and bond-forming methods.
The starter units are nucleosides in which
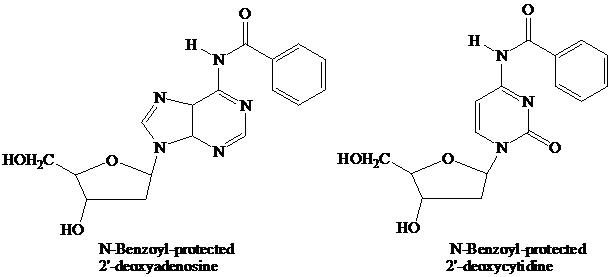
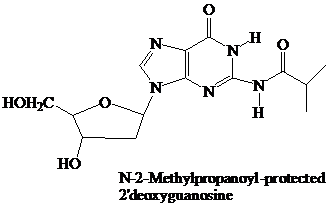
Thymidine lacks an
These
The
(DMT) ether.
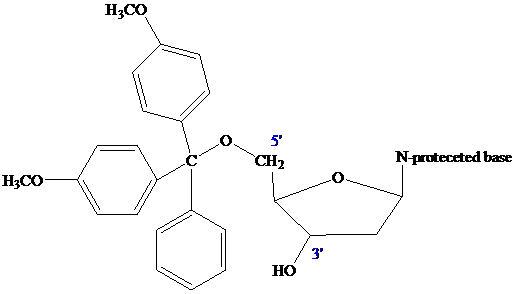
The nucleoside that is to serve as the
controlled-pore glass (CPG) bead by ester formation between its unprotected

The stage is now set for adding the second nucleoside. The four blocked nucleosides prepared
earlier are converted to their corresponding
derivatives. An appropriate A, C, T, or G phosphoramidite is used in each successive stage of the elongation cycle.
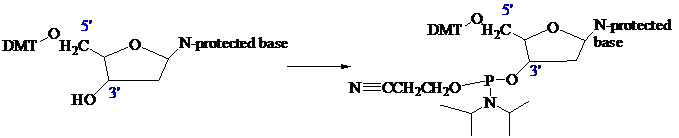
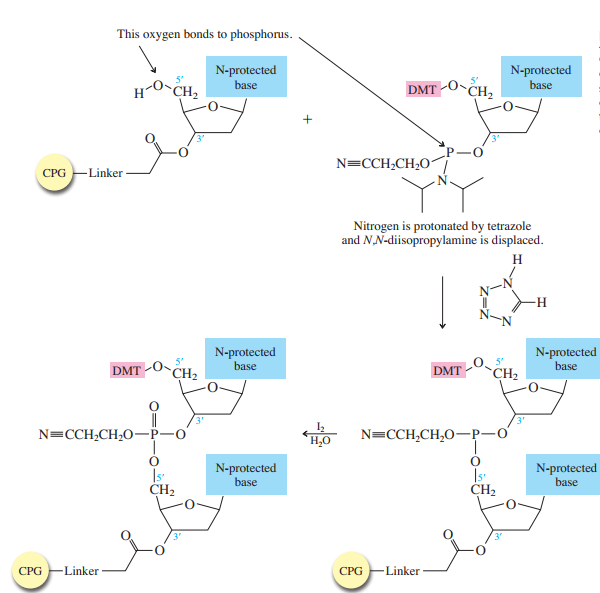
Each phosphoramidite is coupled to the anchored nucleoside by a reaction in which the free
The product of the coupling is a phosphite; it has the general formula
in the last step of Figure
The

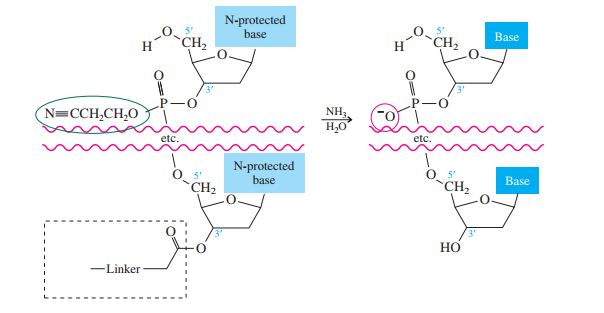
Once all the nucleosides are in place and the last DMT is removed, treatment with aqueous
ammonia removes the acyl and cyanoethyl groups and cleaves the oligonucleotide from the CPG
support.
Structure 1 is the one given for tetrazole in Figure
same molecular formula
structures related?
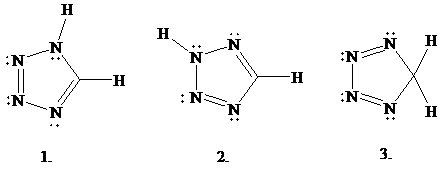
A.
B.
C.
D.
Section 27.6 Many important compounds contain two or more nucleotides joined together by
a phosphodiester linkage. The best known are those in which the phosphodiester joins the
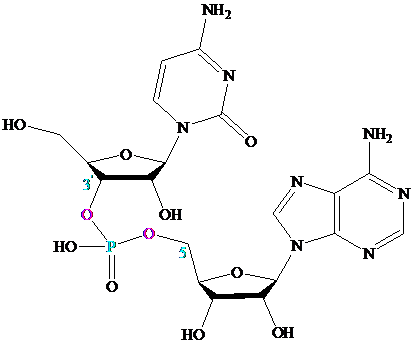
Oligonucleotides contain about
phosphodiester links; polynucleotides can contain thousands of nucleotides.
Want to see the full answer?
Check out a sample textbook solution
Chapter 27 Solutions
Organic Chemistry - Standalone book
- Choose the best reagent for carrying out the following reactions from the list below. Place the letter of the reagent(s) in the box over the reaction arrow. Use only one letter per box. OH OH CH CH CH3 CHS CH3 f OH OCH 3 H A. NaH, then CHI B. C. m-ClC6H4COзH D. E. warm H2SO4/H₂O F. G. H₂/Pd H. I. Cl₂, H₂O J. NaOCH3, CH3OH CH3MgBr in ether, then H3O+ Hg(O2CCF3)2, CH3OH PCC, CH2Cl2 LiAlH4 in ether, then H3O+arrow_forwardWhat is the product of the reaction of 2,4-pentanedione with phenylhydrazine?arrow_forwardIn the reaction of naphthalene with CrO3 in acetic acid. Indicate whether a different product is obtained if carried out at 25°C or with heating (A).arrow_forward
- QUESTION: Fill in the answers in the empty green boxes 1. Step 2 2. Step 3 3. Step 4 (SUM) 4. Step 5 (df) (GIVEN) 5. Determine S y/x value *The data values have been provided in the worksheet attached in the first image*arrow_forwardIf the symbol A is placed in a reaction, at what temperature does it take place?arrow_forwardBy malonic or acetylacetic synthesis, synthesize 3-methyl-4-oxopentanoic acid (indicate the formulas of the compounds).arrow_forward
- oalmitic acid is a 16 carbon acid. In a balanced equation, the products of the sponification of tripalmitin (glyceryl tripalmitate are blank.arrow_forwardWrite the esterification reaction mechanism of salicylic acid and acetic acid to produce aspirin (acetylsalicylic acid). Note: salicylic acid will act as the alcoholarrow_forwardWhat type of interaction would you expect between the following R groups in the tertiary structure of a protein? O -CH2-CO and -CH2-CH2-CH2-CH2-NH3+ a. disulfide bonds b. salt bridges c. hydrogen bonds HO abios vist anisinoo tedt bigil s ai loistaslor sale! 10 OUT d. hydrophobic interactions e. peptide bondsarrow_forward
- 4. True or false: This skeletal structure represents a saturated fatty acid. Ini to 0 fale) me OH faistong starrow_forwardBy malonic or acetylacetic synthesis, synthesize 5-Methyl-2-hexanone (with the formulas of the compounds).arrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' by filling in all the empty green boxes *The values are all provided in the first photo attached*arrow_forward

 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





