
ORGANIC CHEMISTRY
6th Edition
ISBN: 9781260826791
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2.7, Problem 29P
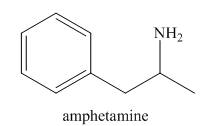
Compounds like amphetamine that contain nitrogen atoms are protonated by the
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Can you please help mne with this problem. Im a visual person, so can you redraw it, potentislly color code and then as well explain it. I know im given CO2 use that to explain to me, as well as maybe give me a second example just to clarify even more with drawings (visuals) and explanations.
Part 1. Aqueous 0.010M AgNO 3 is slowly added to a 50-ml solution containing both carbonate [co32-] = 0.105 M
and sulfate [soy] = 0.164 M anions. Given the ksp of Ag2CO3 and Ag₂ soy below. Answer the ff:
Ag₂ CO3 = 2 Ag+ caq) + co} (aq)
ksp = 8.10 × 10-12
Ag₂SO4 = 2Ag+(aq) + soy² (aq) ksp = 1.20 × 10-5
a) which salt will precipitate first?
(b)
What % of the first anion precipitated will remain in the solution.
by the time the second anion starts to precipitate?
(c) What is the effect of low pH (more acidic) condition on the separate of the carbonate and
sulfate anions via silver precipitation? What is the effect of high pH (more basic)? Provide appropriate
explanation per answer
Part 4. Butanoic acid (ka= 1.52× 10-5) has a partition coefficient of 3.0 (favors benzene) when distributed bet.
water and benzene. What is the formal concentration of butanoic acid in each phase when
0.10M aqueous butanoic acid is extracted w❘ 25 mL of benzene
100 mL of
a) at pit 5.00
b) at pH 9.00
Chapter 2 Solutions
ORGANIC CHEMISTRY
Ch. 2.1 - a. Which compounds are Bronsted-Lowry acids:...Ch. 2.2 - a. Draw the conjugate acid of each base:...Ch. 2.2 - Label each statement as True or False.
a. is the...Ch. 2.2 - Decide which compound is the acid and which is the...Ch. 2.2 - Draw the products formed from the acid-base...Ch. 2.3 - Which compound in each pair is the stronger acid?...Ch. 2.3 - Use a calculator when necessary to answer the...Ch. 2.3 - Rank the conjugate bases of each of group of acids...Ch. 2.3 - Problem-2.10 Considers two acids: (formic acid,)...Ch. 2.3 - Prob. 11P
Ch. 2.4 - Draw the products of each reaction and determine...Ch. 2.4 - Prob. 13PCh. 2.5 - Without reference to a pKa table, decide which...Ch. 2.5 - Rank the labeled H atoms in the following compound...Ch. 2.5 - Which hydrogen in pseudoephedrine, the nasal...Ch. 2.5 - Which compound in each pair is the stronger acid?...Ch. 2.5 - Glycolic acid, HOCH2CO2H, is the simplest member...Ch. 2.5 - Explain the apparent paradox. HBr is a stronger...Ch. 2.5 - The CH bond in acetone, (CH3)2C=O, has a pKa of...Ch. 2.5 - Prob. 23PCh. 2.5 - For each pair of compounds: [1] Which indicated H...Ch. 2.5 - Rank the compounds in each group in order of...Ch. 2.5 - Prob. 26PCh. 2.5 - Prob. 27PCh. 2.6 - Prob. 28PCh. 2.7 - Problem 2.29
Compounds like amphetamine that...Ch. 2.8 - Problem 2.30 Which species are Lewis bases?
a. b....Ch. 2.8 - Which species are Lewis acids?
a. b. c. d.
Ch. 2.8 - For each reaction, label the Lewis acid and base....Ch. 2.8 - Prob. 33PCh. 2.8 - Prob. 34PCh. 2.8 - Label the Lewis acid and base. Use curved arrow...Ch. 2 - 2.36 Propranolol is an antihypertensive agent—that...Ch. 2 - 2.37 Amphetamine is a powerful stimulant of the...Ch. 2 - 2.38 What is the conjugate acid of each base?
a....Ch. 2 - 2.39 What is the conjugate base of each acid?
a....Ch. 2 - Draw the products of each proton transfer...Ch. 2 - Prob. 43PCh. 2 - What is Ka for each compound? Use a calculator...Ch. 2 - What is the pKa for each compound? a. b. c.Ch. 2 - Which of the following bases are strong enough to...Ch. 2 - Draw the products of each reaction. Use the pKa...Ch. 2 - a. What is the conjugate acid of A? b. What is the...Ch. 2 - Dimethyl ether (CH3OCH3) and ethanol (CH3CH2OH)...Ch. 2 - 2.59 Atenolol is a (beta) blocker, a drug used to...Ch. 2 - 2.60 Use the principles in Section 2.5 to label...Ch. 2 - 2.61 Label the three most acidic hydrogen atoms in...Ch. 2 - Prob. 66PCh. 2 - 2.63 Classify each compound as a Lewis base, a...Ch. 2 - 2.64 Classify each species as a Lewis acid, a...Ch. 2 - Label the Lewis acid and Lewis base in each...Ch. 2 - 2.66 Draw the products of each Lewis acid-base...Ch. 2 - Prob. 71PCh. 2 - 2.68 Answer the following questions about the four...Ch. 2 - Prob. 73PCh. 2 - 2.70 Hydroxide can react as a Brønsted-Lowry base...Ch. 2 - 2.71 Answer the following questions about esmolol,...Ch. 2 - Prob. 76PCh. 2 - Prob. 77PCh. 2 - Prob. 82P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate activation energy (Ea) from the following kinetic data: Temp (oC) Time (s) 23.0 180. 32.1 131 40.0 101 51.8 86.0 Group of answer choices 0.0269 kJ/mole 2610 kJ/mole 27.6 kJ/mole 0.215 kJ/mole 20.8 kJ/molearrow_forwardCalculate activation energy (Ea) from the following kinetic data: Temp (oC) Time (s) 23.0 180. 32.1 131 40.0 101 51.8 86.0 choices: 0.0269 kJ/mole 2610 kJ/mole 27.6 kJ/mole 0.215 kJ/mole 20.8 kJ/molearrow_forwardCalculate activation energy (Ea) from the following kinetic data: Temp (oC) Time (s) 23.0 180. 32.1 131 40.0 101 51.8 86.0arrow_forward
- Please solvearrow_forwardRank the compounds in each group below according to their reactivity toward electrophilic aromatic substitution (most reactive = 1; least reactive = 3). Place the number corresponding to the compounds' relative reactivity in the blank below the compound. a. CH₂F CH3 F b. At what position, and on what ring, is bromination of phenyl benzoate expected to occur? Explain your answer. :0: C-O phenyl benzoate 6.Consider the reaction below to answer the following questions. A B C NO₂ FeBr3 + Br₂ D a. The nucleophile in the reaction is: BODADES b. The Lewis acid catalyst in the reaction is: C. This reaction proceeds d. Draw the structure of product D. (faster or slower) than benzene.arrow_forwardPart 2. A solution of 6.00g of substance B in 100.0mL of aqueous solution is in equilibrium, at room temperature, wl a solution of B in diethyl ether (ethoxyethane) containing 25.0 g of B in 50.0 mL 9) what is the distribution coefficient of substance B b) what is the mass of B extracted by shaking 200 ml of an aqueous solution containing 10g of B with call at room temp): i) 100 mL of diethyl ether ii) 50ml of diethyl ether twice iii) 25ml of diethyl ether four timesarrow_forward
- - Rank the following groups of compounds from most acidic (1) to least acidic (4). Place the number corresponding to the compound's relative rank in the blank below the structure. a. NO₂ NO₂ CH2CH2CH2CH2OH CH3 CH3CH2CHOH CH3CH2CH2CH2OH NO₂ CH3CHCH2CH2OH b. OH OH CH₂OH CO₂H HC CN CN CNarrow_forwardGive the major organic product(s) of the following reactions or sequences of reactions. Show all relevant stereochemistry a. H MgBr 1. ether 2. H₂O* 4 COH b. 1. LIAIH, ether 2. H₂O Choose the best reagent(s) for carrying out the following conversions from the list provided below. Place the letter of the best choice in the blank to the left of the conversion. Reagents may be used more than once. a. 1. CH3MgBr, ether 2. H3O+ NaOH b. 1. PBr3 2. C. 2. 1. (CH3)3SiCl, (CH3CH2)3N CH3MgBr, ether 3. H₂O*+ 2. H3O+ e. 1. p-TosCl, pyridine f. نها g. 2. NaOH CrO3, H₂SO4, H₂O 1. NaBH4, ethanol 2. H30* h. PCC, CH2Cl2 Ovoldo-6 a. b. OH OH H OH O any organicarrow_forwardDetermine the rate law for sodium thiosulfate from the following data: [Na2S2O3] Time (s) 0.0318 230. 0.0636 57.5arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning
Types of Matter: Elements, Compounds and Mixtures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=dggHWvFJ8Xs;License: Standard YouTube License, CC-BY