
(a)
Interpretation:
The synthesis of given compound from the given starting material is to be stated.
Concept Introduction:
Amino group reacts faster with the
Answer to Problem 27.71AP
The synthesis of given compound from the given starting material is shown below.
Explanation of Solution
The given reaction is shown below.
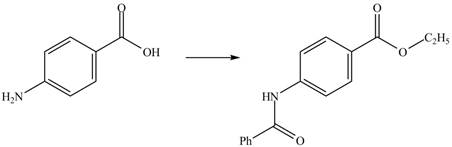
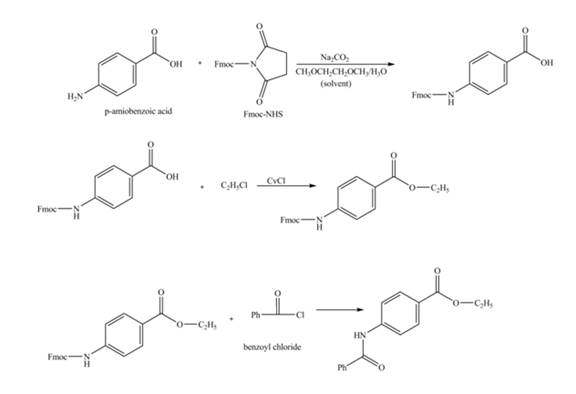
Figure 1
The amine group of the
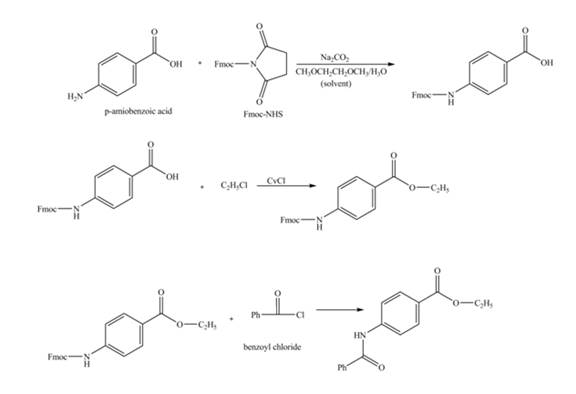
Figure 2
The complete synthesis of the given compound is shown in Figure 2.
(b)
Interpretation:
The synthesis of given compound from the given starting material is to be stated.
Concept Introduction:
Sodium borohydride is a strong reducing agent. The hydride ion of sodium borohydride acts as the nucleophile and gets attached to the carbonyl carbon.
Answer to Problem 27.71AP
The synthesis of given compound from the given starting material is shown below.
Explanation of Solution
The given reaction is shown below.
Figure 3
The benzoic acid reacts with
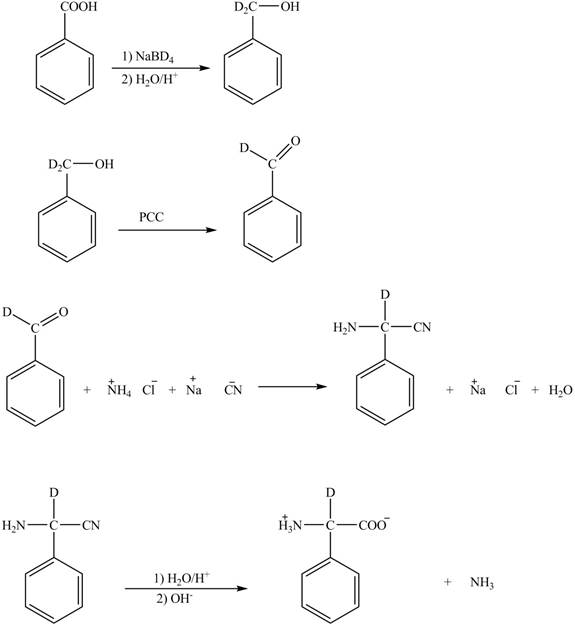
Figure 4
The complete synthesis of the given compound is shown in Figure 4.
(c)
Interpretation:
The synthesis of given compound from the given starting material is to be stated.
Concept Introduction:
Aldehydes reacts with sodium cyanide to form nitrile compound. The nitrile compound reacts with ammonium chloride to form the
Answer to Problem 27.71AP
The synthesis of given compound from the given starting material is shown below.
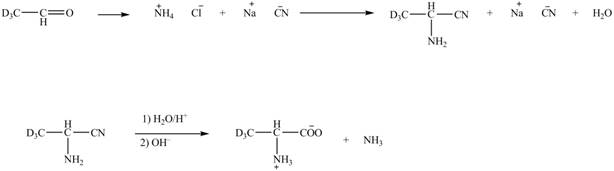
Explanation of Solution
The given reaction is shown below.
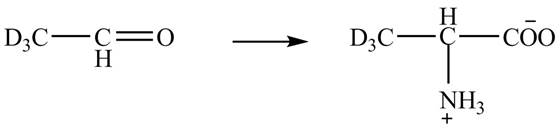
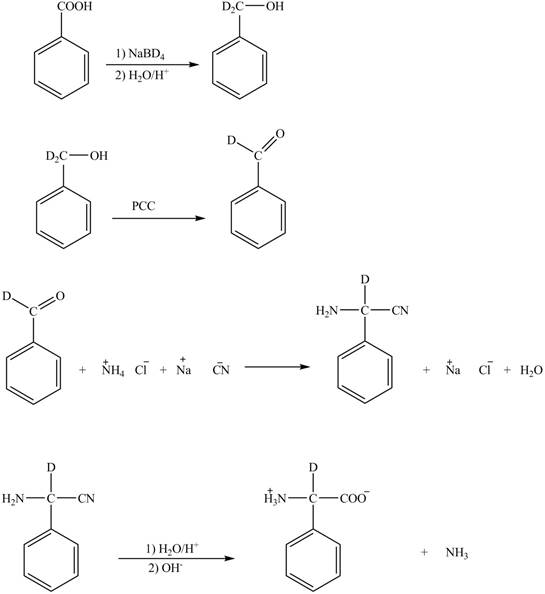
Figure 5
The deuteriated acetaldehyde reacts with ammonium chloride and sodium cyanide to form deuteriated
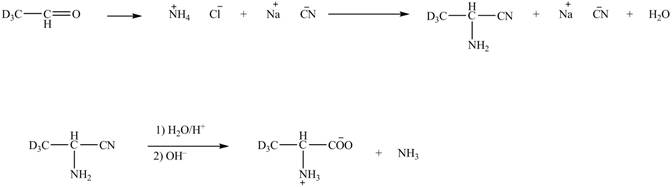
Figure 6
The synthesis of the given compound is shown in Figure 6.
(d)
Interpretation:
The synthesis of given compound from the given starting material is to be stated.
Concept Introduction:
Amino group reacts faster with the alkyl halides than the carboxylic group. The reaction of carboxylic acid can be performed only by protecting the amine group. Fmoc compound is used as the protecting agent for amine group. Amine and acid chloride reacts to form amide bond.
Answer to Problem 27.71AP
The synthesis of given compound from the given starting material is shown below.
Explanation of Solution
The given reaction is shown below.
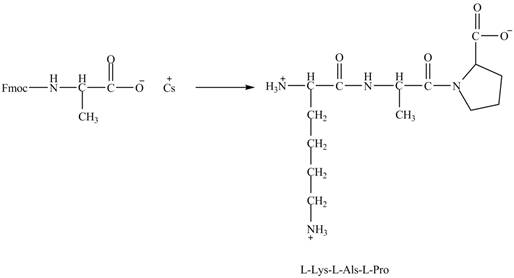
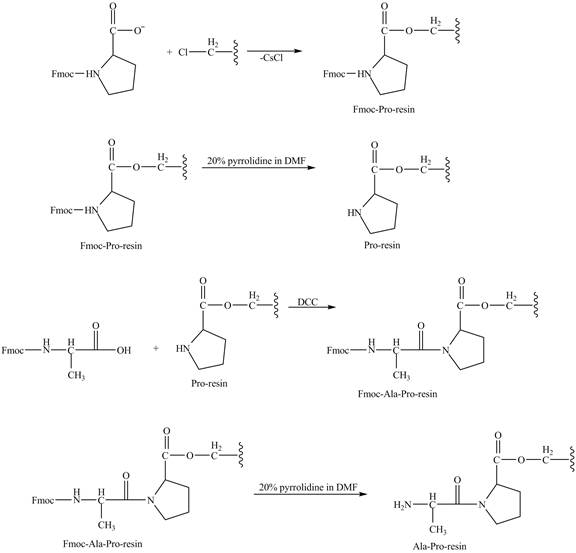
Figure 7
The cesium salt of Fmoc-proline is reacted with the resin linker. The amine is deprotecteed in pyrrolidine. Fmoc-alanine reacts with the prolin resin in presence of
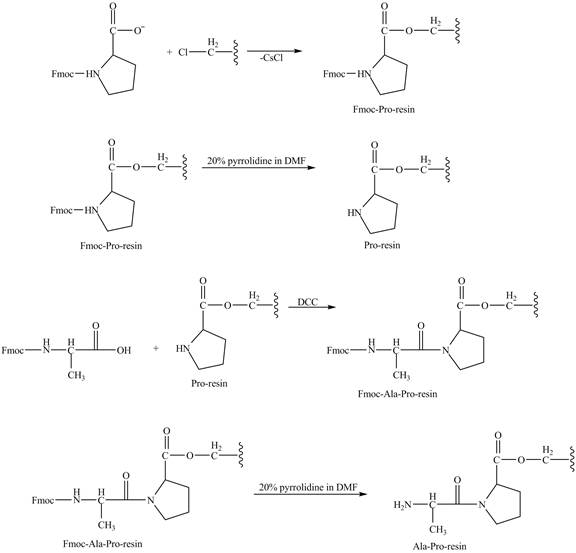
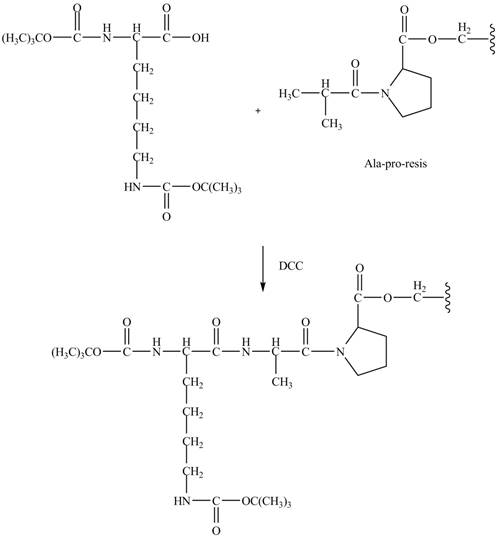
Figure 8
The alanine-proline resin reacts with the Boc protected lysin to form the
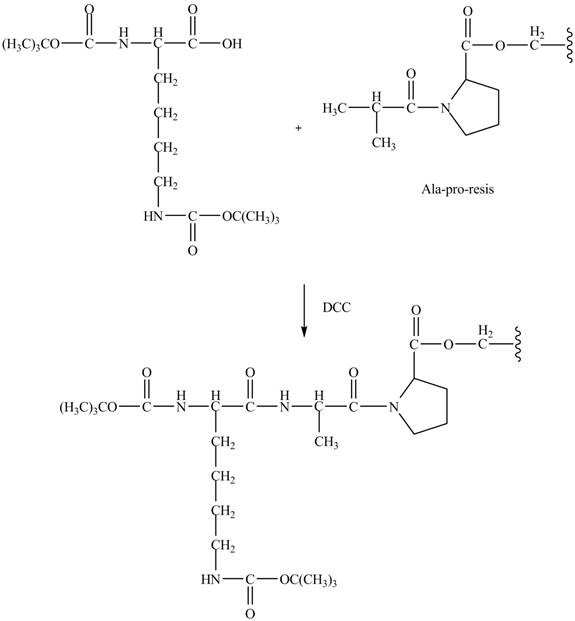
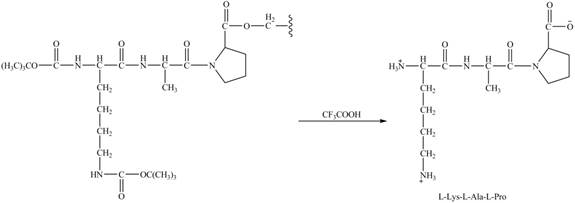
Figure 9
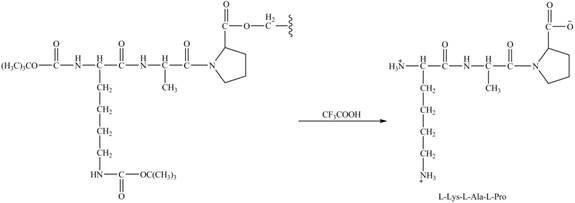 Figure 10
Figure 10
The synthesis of the given compound is shown in Figure 8 and Figure 9 and Figure 10.
(e)
Interpretation:
The synthesis of given compound from the given starting material is to be stated.
Concept Introduction:
Aldehydes reacts with sodium cyanide to form nitrile compound. The nitrile compound reacts with ammonium chloride to form the
Answer to Problem 27.71AP
The complete synthesis of given compound is shown below.
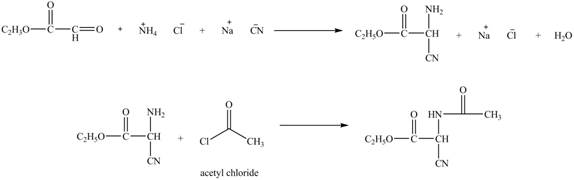
Explanation of Solution
The given reaction is shown below.
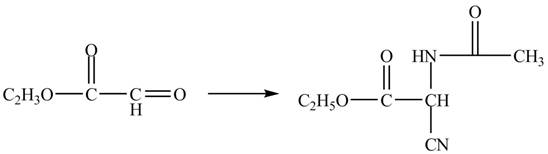
Figure 11
The compound
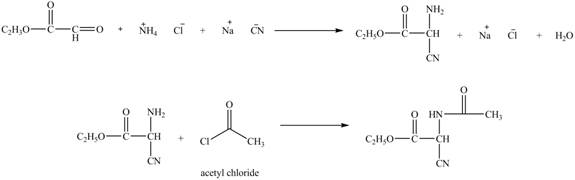
Figure 12
The synthesis of the given compound is shown in Figure 12.
(f)
Interpretation:
The synthesis of given compound from the given starting material is to be stated.
Concept Introduction:
The
Answer to Problem 27.71AP
The complete synthesis of given compound is shown below.
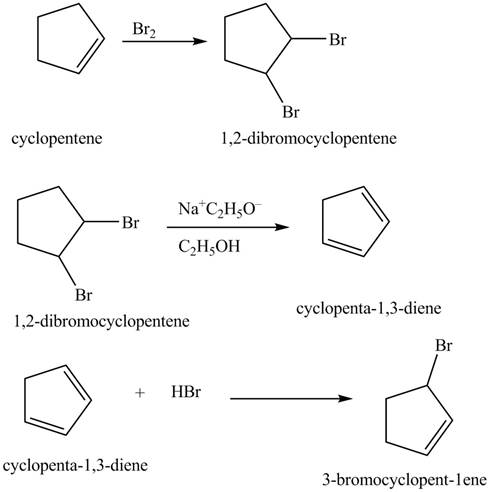
Explanation of Solution
The given reaction is shown below.
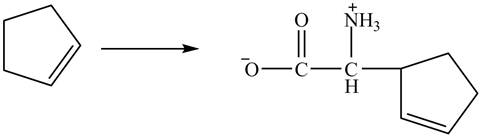
Figure 13
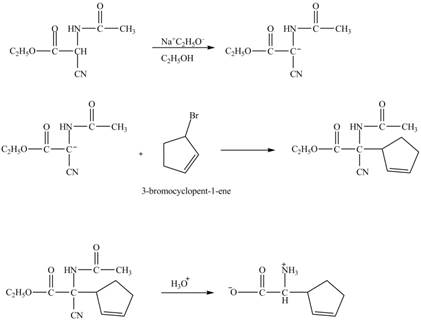
The compound cyclopentene undergo bromination reaction to form
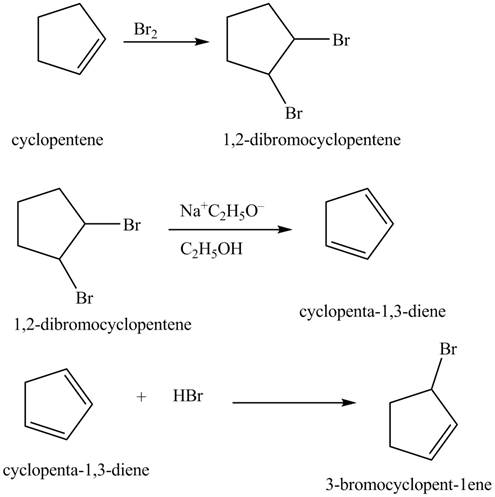
Figure 14
The given nitrile compound upon reaction with sodium ethoxide forms anion. The formed anion reacts with
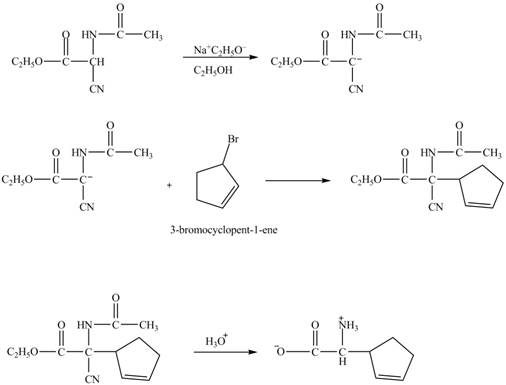
Figure 15
The complete synthesis of given compound is shown in Figure 14 and Figure 15.
(g)
Interpretation:
The synthesis of given compound from the given starting material is to be stated.
Concept Introduction:
The reaction of carboxylic acid and amines to form amide is not possible. Amines are very basic so it abstracts acidic proton from the carboxylic acid. The reaction is performed in the presence of
Answer to Problem 27.71AP
The complete synthesis of given compound is shown below.
Explanation of Solution
The given reaction is shown below.
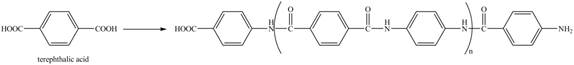
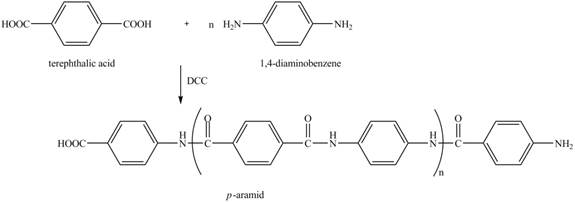
Figure 16
The reaction between terephthalic acid and

Figure 17
The synthesis of given compound is shown in Figure 17.
Want to see more full solutions like this?
Chapter 27 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- What alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. and two equivalents of CH2=O draw structure ...arrow_forwardH-Br Energy 1) Draw the step-by-step mechanism by which 3-methylbut-1-ene is converted into 2-bromo-2-methylbutane. 2) Sketch a reaction coordinate diagram that shows how the internal energy (Y- axis) of the reacting species change from reactants to intermediate(s) to product. Brarrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 H-CI CH2Cl2 CIarrow_forward
- Draw the products of the stronger acid protonating the other reactant. དའི་སྐད”“ H3C OH H3C CH CH3 KEq Product acid Product basearrow_forwardDraw the products of the stronger acid protonating the other reactant. H3C NH2 NH2 KEq H3C-CH₂ 1. Product acid Product basearrow_forwardWhat alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. draw structure ... andarrow_forward
- Draw the products of the stronger acid protonating the other reactant. H3C-C=C-4 NH2 KEq CH H3C `CH3 Product acid Product basearrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 Br H-Br CH2Cl2 + enant.arrow_forwardDraw the products of the stronger acid protonating the other reactant. KEq H₂C-O-H H3C OH Product acid Product basearrow_forward
- Draw the products of the stronger acid protonating the other reactant. OH KEq CH H3C H3C `CH3 Product acid Product basearrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). Ph H-I CH2Cl2arrow_forward3 attempts left Check my work Draw the products formed in the following oxidative cleavage. [1] 03 [2] H₂O draw structure ... lower mass product draw structure ... higher mass productarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

