
(a)
Interpretation:
Given set of prostaglandins has to be classified.
Concept introduction:
Prostaglandins are synthesized in the body and they are not transported. They perform their functions at the place where they are synthesized itself and are known as local mediators. They are biochemical regulators and are even more powerful than steroids. This is synthesized biologically from arachidonic acid using an enzyme cyclooxygenases.
Classification of prostaglandin is done by following simple rules. Letters “PG” in each case represent prostaglandin and the last letter implies the substitution pattern. Few examples are,
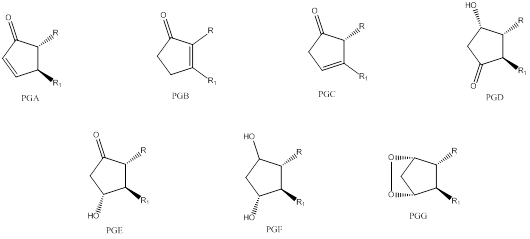
The number of carbon-carbon double bonds in the side chain is indicated by the subscript in the end.
To classify: the given prostaglandin
(b)
Interpretation:
Given set of prostaglandins has to be classified.
Concept introduction:
Prostaglandins are synthesized in the body and they are not transported. They perform their functions at the place where they are synthesized itself and are known as local mediators. They are biochemical regulators and are even more powerful than steroids. This is synthesized biologically from arachidonic acid using an enzyme cyclooxygenases.
Classification of prostaglandin is done by following simple rules. Letters “PG” in each case represent prostaglandin and the last letter implies the substitution pattern. Few examples are,

The number of carbon-carbon double bonds in the side chain is indicated by the subscript in the end.
To classify: the given prostaglandin
Want to see the full answer?
Check out a sample textbook solution
Chapter 26 Solutions
Organic Chemistry, Binder Ready Version
- Which of the following is true for a particular reaction if ∆G° is -40.0 kJ/mol at 290 K and –20.0 kJ/mol at 390 K?arrow_forwardWhat is the major product of the following reaction? O O OH OH 1. BH 2. H₂O₂, NaOH OH OHarrow_forwardDraw the products formed when each ester is hydrolyzed with water and sulfuric acid.arrow_forward
- Draw the products of the hydrolysis reaction between the ester molecule and water. Determine the products of the following reaction.arrow_forwardWhat is the unsaturation number for compounds with the formula C₂H₁₂Cl₂? O õ õ o o 4 3arrow_forwardIndicate the product obtained (formula). F3C. CF3 Br NH2 NH OMe K2CO3, DABCO, DMFarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





