
(a)
Interpretation:
Styrene can auto initiate free radical
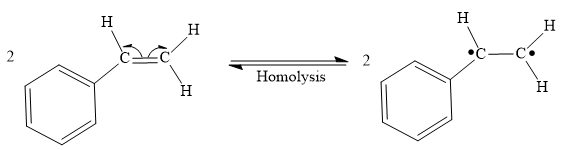
Step 1: Homolysis of the vinyl π bond in a styrene molecule
Step 2: Homolysis of the vinyl π bond in a second styrene molecule
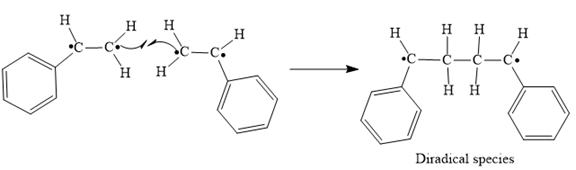
Step 3: Tail-to-tail addition of the radicals from Steps 1 and 2
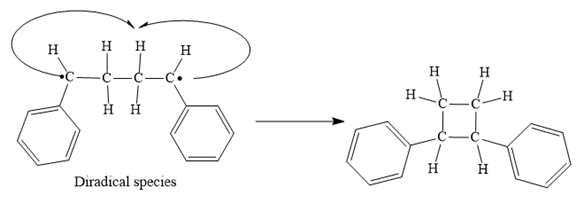
Step 4: Radical coupling of the diradical formed in Step 3.
It as is to be used curved arrow notation to show the steps in a given mechanism.
Concept introduction:
“The breaking of a covalent bond, whereby the electrons making up that bond are distributed equally to the atoms which are disconnected is known as the homolytic bond dissociation or homolysis.” So in homolysis generally radicals are formed. In homolysis, a covalent bond is a breakdown equally and each atom acquires a single electron which is called a radical and a single barbed arrow (![]() ) is used to represents the movement of a single electron in a homolysis process. In homolysis, the radicals from a breaking of the weakest bond of the molecule give the major products. The weakest bond possesses the smallest
) is used to represents the movement of a single electron in a homolysis process. In homolysis, the radicals from a breaking of the weakest bond of the molecule give the major products. The weakest bond possesses the smallest
Answer to Problem 26.74P
The mechanism for the given steps with curved arrow notation is given below:
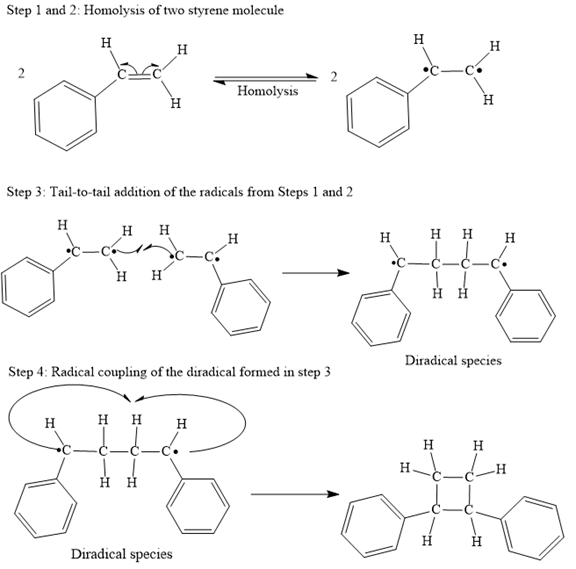
Explanation of Solution
Both steps step 1 and step 2 are same, the homolysis of the vinyl π bond in two styrene molecules.
In step 3 Tail-to-tail addition of the radicals from Steps 1 and 2 takes place as below:
Finally, the radical coupling of the diradical formed in step 3 takes place as below:
A curved arrow notation to show the steps in a given mechanism is used.
(b)
Interpretation:
Styrene can auto initiate free radical polymerization and form polystyrene in the absence of an initiator. At temperatures
Step 1: Homolysis of the vinyl π bond in a styrene molecule
Step 2: Homolysis of the vinyl π bond in a second styrene molecule
Step 3: Tail-to-tail addition of the radicals from Steps 1 and 2
Step 4: Radical coupling of the diradical formed in Step 3.
It is to be explained above steps consistent with those for free radical polymerization.
Concept introduction:
“The breaking of a covalent bond, whereby the electrons making up that bond are distributed equally to the atoms which are disconnected is known as the homolytic bond dissociation or homolysis.” So in homolysis generally radicals are formed. In homolysis, a covalent bond is a breakdown equally and each atom acquires a single electron which is called a radical and a single barbed arrow (![]() ) is used to represents the movement of a single electron in a homolysis process. In homolysis, the radicals from a breaking of the weakest bond of the molecule give the major products. The weakest bond possesses the smallest bond dissociation energy. These radials are propagated in the polymerization to elongate the chain of the polymer called a propagation step.
) is used to represents the movement of a single electron in a homolysis process. In homolysis, the radicals from a breaking of the weakest bond of the molecule give the major products. The weakest bond possesses the smallest bond dissociation energy. These radials are propagated in the polymerization to elongate the chain of the polymer called a propagation step.
Answer to Problem 26.74P
No, this mechanism is not consistent with a free-radical polymerization, because this mechanism not produces a polymer.
Explanation of Solution
No, this mechanism is not consistent with a free-radical polymerization. In polymerization, the propagation step must occur to elongate the chain of the polymer. But in the given mechanism, the propagation step does not occur thus it not forms polymer, actually, it is a dimer.
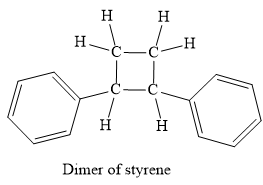
It is explained above steps consistent with those for free radical polymerisation.
(c)
Interpretation:
It is to be explained why the use of an initiator such as benzoyl peroxide more efficient in the synthesis of polystyrene than auto initiated polymerization.
Concept introduction:
“The breaking of a covalent bond, whereby the electrons making up that bond are distributed equally to the atoms which are disconnected is known as the homolytic bond dissociation or homolysis.” So in homolysis generally radicals are formed. In homolysis, a covalent bond is a breakdown equally and each atom acquires a single electron which is called a radical and a single barbed arrow (![]() ) is used to represents the movement of a single electron in a homolysis process. In homolysis, the radicals from a breaking of the weakest bond of the molecule give the major products. The weakest bond possesses the smallest bond dissociation energy. These radials are propagated in polymerization are elongated to grow the chain of the polymer called a propagation step.
) is used to represents the movement of a single electron in a homolysis process. In homolysis, the radicals from a breaking of the weakest bond of the molecule give the major products. The weakest bond possesses the smallest bond dissociation energy. These radials are propagated in polymerization are elongated to grow the chain of the polymer called a propagation step.
Answer to Problem 26.74P
In case of autoinitiator reaction, the reaction can be terminated too rapidly, because the diradicals formed from the two styrene molecule undergoes dimerization to form the 1, 2-phenylcylcobutane as a product, instead of polymerization. Due to the formation of this product, the growth of a long chain of the molecule does not occur. But with benzoyl peroxide, the radical from the initiation step is a monoradical and it will continue the propagation step to build up polymer, through the chain growth.
Explanation of Solution
In case of autoinitiator reaction, the reaction can be terminated too rapidly, because the diradicals formed from the two styrene molecule undergoes dimerization to form the 1, 2-phenylcylcobutane as a product, instead of polymerization. Due to the formation of this product, the growth of a long chain of the molecule does not occur.

But with benzoyl peroxide, the radical from the initiation step is a monoradical and it will continue the propagation step to build up polymer, through the chain growth.
It is explained why the use of an initiator such as benzoyl peroxide more efficient in the synthesis of polystyrene than auto initiated polymerization.
Want to see more full solutions like this?
Chapter 26 Solutions
Get Ready for Organic Chemistry
- Identify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forwardRank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forward
- Rank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forwardWhy isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forward
- What is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forwardComplete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forward
- Q Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forwardb. ὋΗ CH3CH2OH H2SO4arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





