
(a)
Interpretation:
A mechanism for the initiation step for the
Concept introduction:
The
The polymerizations can be initiated by anions or cations, resulting in anionic polymerization or cationic polymerization, respectively. The polymerization involves, 1) initiation, 2) propagation, and 3) termination.
Initiation is the first step of the polymerization process. An active center is created during initiation, from which a polymer chain is generated. Not all monomers are susceptible to all types of initiators.
In propogation a reactive intermediate is repetitively redeveloped throughout the sequence of a chemical chain reaction. Then it is terminated by adding mono
Answer to Problem 26.42P
The initiation step for the polymerization reaction of the given compound is as follows.
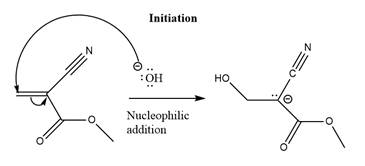
Explanation of Solution
The
The given explanation is obtained by applying the concept polymerization mechanism.
(b)
Interpretation:
A mechanism for the propagation step for the polymerization reaction of the given compound is to be determined.
Concept introduction:
The chemical reaction in which the monomer molecules reacts together to form polymer chains or three dimentional networks is termed as polymerization. This are classified by different syatem avaible for the different types if polymerization.
The polymerizations can be initiated by anions or cations, resulting in anionic polymerization or cationic polymerization, respectively. The polymerization involves, 1) initiation, 2) propagation, and 3) termination.
Initiation is the first step of the polymerization process. An active center is created during initiation, from which a polymer chain is generated. Not all monomers are susceptible to all types of initiators.
In propogation a reactive intermediate is repetitively redeveloped throughout the sequence of a chemical chain reaction. Then it is terminated by adding mono functional groups that having equal of different types of monomer.
Answer to Problem 26.42P
The propagation step for the polymerization reaction of the given compound is as follows.
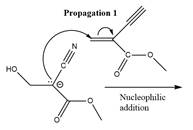
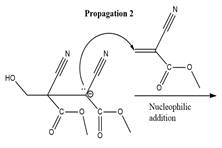
Explanation of Solution
The carbanion formed in initiation step reacts with another equivalent of
in the first nucleophilic addition step. The propagation step repeats the nucleophilic addition reaction.
The given explanation is obtained by applying the concept polymerization mechanism.
(c)
Interpretation:
The structure of
Concept introduction:
The chemical reaction in which the monomer molecules reacts together to form polymer chains or three dimentional networks is termed as polymerization. This are classified by different syatem avaible for the different types if polymerization.
The polymerizations can be initiated by anions or cations, resulting in anionic polymerization or cationic polymerization, respectively. The polymerization involves, 1) initiation, 2) propagation, and 3) termination.
Initiation is the first step of the polymerization process. An active center is created during initiation, from which a polymer chain is generated. Not all monomers are susceptible to all types of initiators.
In propogation a reactive intermediate is repetitively redeveloped throughout the sequence of a chemical chain reaction. Then it is terminated by adding mono functional groups that having equal of different types of monomer.
Answer to Problem 26.42P
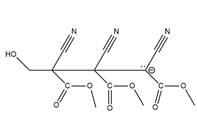
The structure of
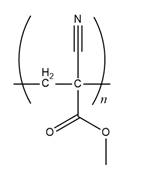
Explanation of Solution
The propagation step repeats the nucleophilic addition reaction to produce
The given explanation is obtained by applying the concept polymerization mechanism.
(d)
Interpretation:
Why is
Concept introduction:
The chemical reaction in which the monomer molecules reacts together to form polymer chains or three dimentional networks is termed as polymerization. This are classified by different syatem avaible for the different types if polymerization.
The polymerizations can be initiated by anions or cations, resulting in anionic polymerization or cationic polymerization, respectively. The polymerization involves, 1) initiation, 2) propagation, and 3) termination.
Initiation is the first step of the polymerization process. An active center is created during initiation, from which a polymer chain is generated. Not all monomers are susceptible to all types of initiators.
In propogation a reactive intermediate is repetitively redeveloped throughout the sequence of a chemical chain reaction. Then it is terminated by adding mono functional groups that having equal of different types of monomer.
Answer to Problem 26.42P
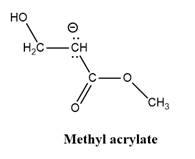
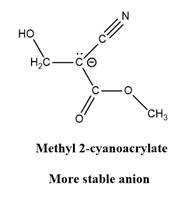
Explanation of Solution
The given explanation is obtained by applying the concept polymerization mechanism, resonance and inductive effect.
(e)
Interpretation:
Why or why not methyl 2-cyanoacrylate would be a good candidate for cationic polymerization is to be explained.
Concept introduction:
The chemical reaction in which the monomer molecules reacts together to form polymer chains or three dimentional networks is termed as polymerization. This are classified by different syatem avaible for the different types if polymerization.
The polymerizations can be initiated by anions or cations, resulting in anionic polymerization or cationic polymerization, respectively. The polymerization involves, 1) initiation, 2) propagation, and 3) termination.
Initiation is the first step of the polymerization process. An active center is created during initiation, from which a polymer chain is generated. Not all monomers are susceptible to all types of initiators.
In propogation a reactive intermediate is repetitively redeveloped throughout the sequence of a chemical chain reaction. Then it is terminated by adding mono functional groups that having equal of different types of monomer.
Answer to Problem 26.42P
Explanation of Solution
The given explanation is obtained by applying the concept polymerization mechanism.
Want to see more full solutions like this?
Chapter 26 Solutions
Get Ready for Organic Chemistry
- Assign these COSY Spectrumarrow_forwardAssign these C-NMR and H-NMR Spectrumarrow_forwardPredict the product of this organic reaction: IZ + HO i P+H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. No Answer Click and drag to start drawing a structure. ☐ :arrow_forward
- Predict the products of this organic reaction: 0 O ----- A + KOH ? CH3-CH2-C-O-CH2-C-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. X ⑤ èarrow_forwardPredict the products of this organic reaction: O CH3 + H2O + HCI A A? CH3-CH2-C-N-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forwardWhat is the missing reactant in this organic reaction? R+ HO-C-CH2-CH3 0= CH3 CH3 —CH, C−NH—CH CH3 + H₂O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. No Answer Click anywhere to draw the first atom of your structure. €arrow_forward
- 个 CHEM&131 9267 - $25 - Intro to Mail - Hutchison, Allison (Student x Aktiv Learnin https://app.aktiv.com Draw the product of the reaction shown below. Ignore inorganic byproducts. + Na2Cr2O7 Acetone, H2SO4 Type here to search Dryng OH W Prarrow_forwardPredict the products of this organic reaction: OH + NaOH A? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click and drag to start drawing a structure. ✓ Sarrow_forwardPredict the products of this organic reaction: CH3-C-O-CH2-CH2-C-CH3 + H₂O ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. :☐ darrow_forward
- DE d. Draw an arrow pushing mechanism for the following IN O CI N fo 人 P Polle DELL prt sc home end ins F5 F6 F7 F8 F9 F10 F11 F12arrow_forwardPredict the products of this organic reaction: + H₂O H* ? A Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click and drag to start drawing a structure.arrow_forwardPredict the major organic products of the reaction below and draw them on right side of the arrow. If there will be no significant reaction, check the box below the drawing area instead. C Cl CH, OH There will be no significant reaction. + pyridine G Click and drag to start drawing a structure.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





