
(a)
Interpretation:
Monomer shown here can undergo free radical
Concept introduction:
The first step of free radical polymerization is initiation. Homolysis of benzoyl peroxide takes place in the first step. Benzoyl peroxide is used as a radical initiator. Homolysis of benzoyl peroxide is further promoted by the additional resonance stabilization in the resulting radical. After initiation, propagation occurs via radical addition. In first step propagation, the initial radical adds to the C=C bond of the monomer giving a new radical. The new radical reacts with the other molecule of monomer in the second propagation. From the growing polymer chain, one can identify the structural pattern that is repeated which is the condensed formula showing repeating unit. The name of the polymer is written by writing poly followed by the monomer’s name, and we can enclose the monomer’s name in parenthesis as it consists of two or more words.
Answer to Problem 26.33P
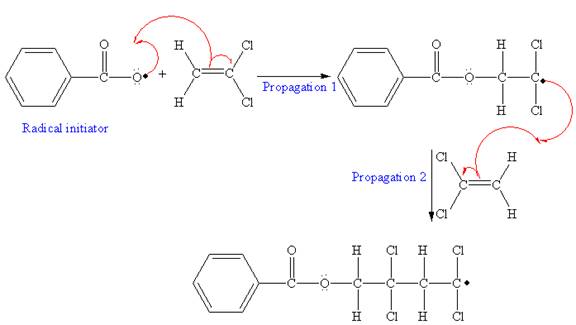
The mechanism for the first two propagation steps using benzoyl peroxide as the initiator is shown below:
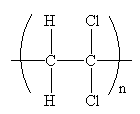
The condensed formula for the polymer showing the repeating unit is shown below:
A name for the polymer is: Poly(vinylidene chloride)
Explanation of Solution
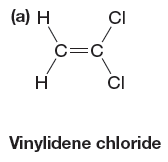
The given monomer is
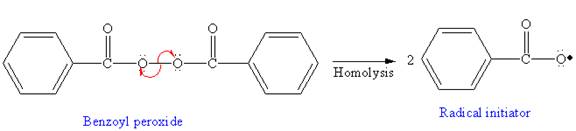
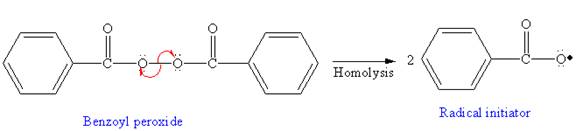
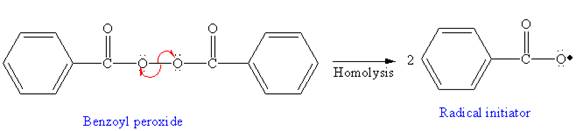
In the initiation step, homolysis of benzoyl peroxide takes place to form a radical initiater as follows:
After initiation, propagation occurs via radical addition. In first step propagation, the initial radical adds to the C=C bond of the monomer giving a new radical. The new radical reacts with the other molecule of the monomer in the second propagation. The mechanism for the first two propagation steps is shown below:
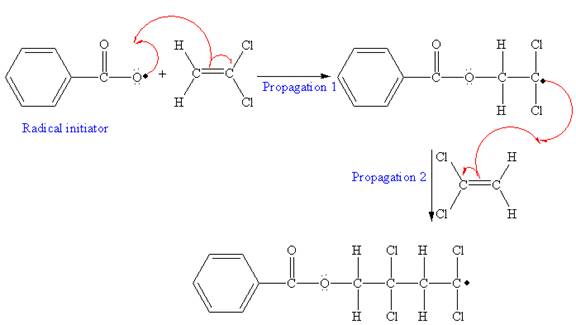
The condensed formula showing repeating unit is shown below:

A name for the polymer is written by writing poly- followed by the monomer’s name, and we can enclose the monomer’s name in parenthesis as it consists of two or more words. Therefore, the name of the polymer is: Poly(vinylidene chloride).
The mechanism for the first two propagation steps for the given monomer is drawn on the basis of chain-growth polymerization. The condensed formula is drawn identifying the structural pattern that is repeated in the growing chain.
(b)
Interpretation:
Monomer shown here can undergo free radical polymerization. For its polymerization: the mechanism for the first two propagation steps using benzoyl peroxide as the initiator is to drawn. The condensed formula for the polymer showing the repeating unit is to be drawn, and a name for the polymer is to be provided.
Concept introduction:
The first step of free radical polymerization is initiation. Homolysis of benzoyl peroxide takes place in first step. Benzoyl peroxide is used as a radical initiator. Homolysis of benzoyl peroxide is further promoted by the additional resonance stabilization in the resulting radical. After initiation, propagation occurs via radical addition. In first step propagation, the initial radical adds to the C=C bond of the monomer giving a new radical. The new radical reacts with the other molecule of the monomer in the second propagation. From the growing polymer chain, one can identify the structural pattern that is repeated which is the condensed formula showing the repeating unit. The name of the polymer is written by writing poly followed by the monomer’s name, and we can enclose the monomer’s name in parenthesis as it consists of two or more words.
Answer to Problem 26.33P
The mechanism for the first two propagation steps using benzoyl peroxide as the initiator is shown below:
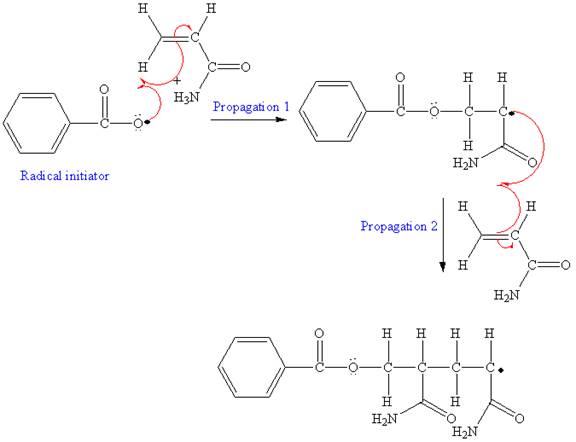
The condensed formula for the polymer showing the repeating unit is shown below:
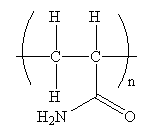
A name for the polymer is: Polyacrylamide.
Explanation of Solution
The given monomer is,
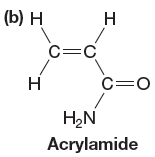
In the initiation step, homolysis of benzoyl peroxide takes place to form a radical initiator as follows:
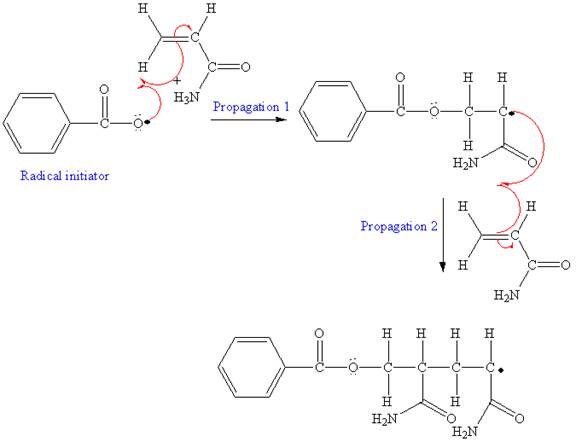
After initiation, propagation occurs via radical addition. In first step propagation, the initial radical adds to the C=C bond of the monomer giving a new radical. The new radical reacts with the other molecule of the monomer in the second propagation. The mechanism for the first two propagation steps is shown below:
The condensed formula showing repeating unit is shown below:

A name for the polymer is written by writing poly- followed by the monomer’s name, and we can enclose the monomer’s name in parenthesis as it consists of two or more words. Therefore, the name of the polymer is: Polyacrylamide.
The mechanism for the first two propagation steps for given monomer are drawn on the basis of chain-growth polymerization. The condensed formula is drawn identifying the structural pattern that is repeated in the growing chain.
(c)
Interpretation:
Monomer shown here can undergo free radical polymerization. For its polymerization: the mechanism for the first two propagation steps using benzoyl peroxide as the initiator is to drawn. The condensed formula for the polymer showing the repeating unit is to be drawn, and a name for the polymer is to be provided.
Concept introduction:
The first step of free radical polymerization is initiation. Homolysis of benzoyl peroxide takes place in first step. Benzoyl peroxide is used as a radical initiator. Homolysis of benzoyl peroxide is further promoted by the additional resonance stabilization in the resulting radical. After initiation, propagation occurs via radical addition. In first step propagation, the initial radical adds to the C=C bond of the monomer giving a new radical. The new radical reacts with the other molecule of the monomer in the second propagation. From the growing polymer chain, one can identify the structural pattern that is repeated which is the condensed formula showing the repeating unit. The name of the polymer is written by writing poly followed by the monomer’s name, and we can enclose the monomer’s name in parenthesis as it consists of two or more words.
Answer to Problem 26.33P
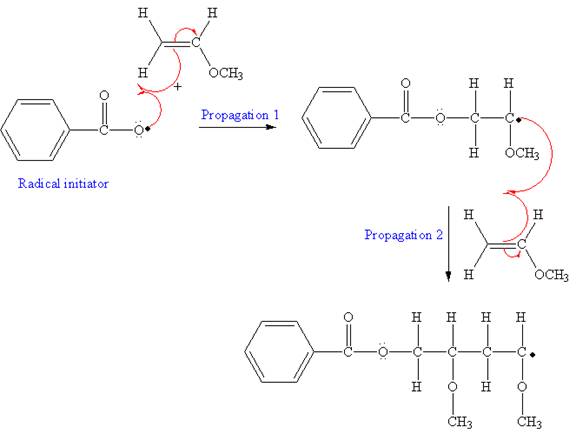
The mechanism for the first two propagation steps, using benzoyl peroxide as the initiator is shown below:
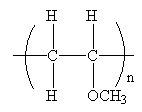
The condensed formula for the polymer showing the repeating unit is shown below:
A name for the polymer is: Poly(methyl vinyl ether).
Explanation of Solution
The given monomer is
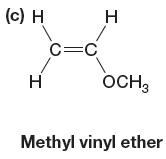
In the initiation step, the homolysis of benzoyl peroxide takes place to form radical initiator as follows:
After initiation, propagation occurs via radical addition. In first step propagation, the initial radical adds to the C=C bond of the monomer giving a new radical. The new radical reacts with the other molecule of monomer in the second propagation. The mechanism for the first two propagation steps is shown below:

The condensed formula showing repeating unit is shown below:

A name for the polymer is written by writing poly- followed by the monomer’s name, and we can enclose the monomer’s name in parenthesis as it consists of two or more words. Therefore, the name of the polymer is: Poly(methyl vinyl ether).
The mechanism for the first two propagation steps for the given monomer is drawn on the basis of chain-growth polymerization. The condensed formula is drawn identifying the structural pattern that is repeated in the growing chain.
Want to see more full solutions like this?
Chapter 26 Solutions
Organic Chemistry: Principles And Mechanisms (second Edition)
- PLEASE READ!!! I DONT WANT EXAMPLES, I DONT WANT WORDS OR PARAGRAPHS!!! PLEASE I UNDERSTAND THE BASICS BUT THIS IS AN EXCEPTION THAT EVEN THE INTERNET CANT HELP!!!! THIS IS THE THIRD TIME I'VE SENT THOSE QUESTIONS SO PLEASE DONT RESEND THE SAME STUFF, ITS NOT HELPING ME!!! I ALSO ALREADY TRIED TO DRAW THE MECHANISM MYSELF, SO IF ITS RIGHT PLEASE TELL ME OR TELL ME WHAT I HAVE TO CHANGE!!! First image: I have to SHOW (DRAWING) the mechanism (with arows and structures of molecules) NOT WORDS PLEASE! of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primary Second image: I have to show the mechanism (IMAGE) (with arrows and structures of the molecules) NOT WORDS PLEASE !! for the reaction on the left, where the alcohol A is added fast in one portion HOMEWORK, NOT EXAM!! ALL DETAILS ARE IN THE IMAGES PLEASE LOOK AT THE IMAGES, DONT LOOK AT THE AI GENERATED TEXT!!!arrow_forwardWrite the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M+ = 85.0899. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 1.008 12C 98.90 12.000 14N 99.63 14.003 160 99.76 15.995 Molecular formula (In the order CHNO, with no subscripts)arrow_forwardUse the data below from an electron impact mass spectrum of a pure compound to deduce its structure. Draw your structure in the drawing window. Data selected from the NIST WebBook, https://webbook.nist.gov/chemistry/ m/z Relative intensity 59 3.0 58 64 43 100 15 23 • You do not have to consider stereochemistry. •You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. + n[] 85 // ? CH4 Previous Nextarrow_forward
- Write the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M* = 128.0632. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 12C 98.90 14N 99.63 160 99.76 Molecular formula 1.008 12.000 14.003 15.995 (In the order CHNO, with no subscripts)arrow_forwardCan I please get help with this? And can I please the lowest possible significant number?arrow_forwardWhat is the molar mass of a gas that takes three times longer to effuse than helium?arrow_forward
- First image: I have to show the mecanism (with arows and structures) of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primary Second image: I have to show the mecanism (with arrows and structures) for the reaction on the left, where the alcohol A is added fast in one portion its not an examarrow_forwardwhat is the skeletal structure of a tertiary alkyl fluoride with six carbon atoms and no rings.arrow_forwardOne step of glycolysis is a retro-aldol reaction (aldolase) to produce ATP.Below is the aldol reaction of the equilibrium. Show the mechanism for the base catalyzed reaction. *see imagearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





