
Combine the following structures to create a ribonucleotide. Show where water is removed to form an N-glycosidic linkage and where water is removed to form a phosphate ester. Draw the resulting ribonucleotide structure, and name it.
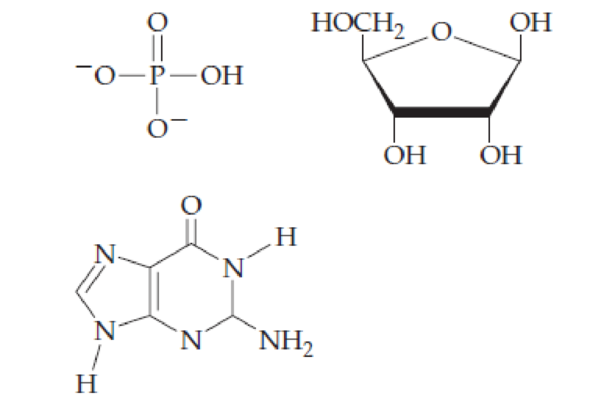
Interpretation:
The ribonucleotide structure has to be predicted and named.
Concept Introduction:
Composition of nucleic acid: Nucleic acid is a polymer of nucleotides. Each nucleotide has three parts: a sugar, a nitrogenous base, and a phosphate group.
Sugar: In both DNA and RNA, sugar portion is found. In DNA, the sugar is D-ribose, where at 2’hydroxyl group is absent and in RNA, the hydroxyl group is present at 2’.
Nitrogenous bases: Five types of nitrogenous bases (has unique one-letter code A, G, T, U, and C) are derived from two parent compounds called purine and pyrimidine. The purine derivatives are Adenine and Guanine are two fused rings. The pyrimidine derivatives are six-membered nitrogen containing ring. Adenine, Guanine, Thymine, and Cytosine are the nitrogenous bases present in DNA. Adenine, Guanine, Cytosine and Uracil are the nitrogenous bases present in RNA.
Nucleotide: (Nucleoside + phosphate)
Nucleotides are the building blocks of nuclei acids; monomers of DNA and RNA polymers. At carbon-5’ of the ribose sugar, a phosphate group is added which is collectively known as nucleotide. Phosphate groups can be added to any of the nucleotide to form diphosphate or triphosphate.
Nucleoside and its naming: The combination of monosaccharide (sugar) and nitrogenous base is known as nucleoside. The nucleoside names are the nitrogenous base name modified with criteria. While naming nucleoside of purine derivatives the suffix ‘-osine’ is included and for pyrimidine derivatives the suffix ‘-idine’ is used. No prefix used for the nucleosides containing ribose and the prefix ‘deoxy-’ is used for deoxyribose.
Naming nucleotide: At the end of the nucleoside, phosphate group is added. For example, 5’-monophosphate means adding one phosphate group at 5’carbon in the sugar ring.
Numbering the atoms in sugar and base rings:
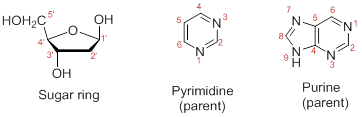
In order to distinguish the atoms in the sugar of a nucleoside and atoms of a base ring, numbers without prime is used for atoms in the base ring and numbers with prime used for the atoms in the sugar ring.
Answer to Problem 26.22UKC
The structure is,
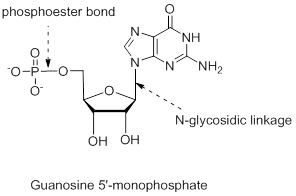
Explanation of Solution
The nucleotide structure is a combination of nucleoside [ribose (presence of –OH group at 2’ carbon atom in the sugar ring) and guanine base (derivative of purine parent)]. The nucleoside name is the nitrogenous name itself; thus, name is Guanine. Looking at the criteria the name of nitrogenous base is modified as follows, the suffix ‘-osine’ is used for the purine derivatives, as here it is Adenine to Guanosine.
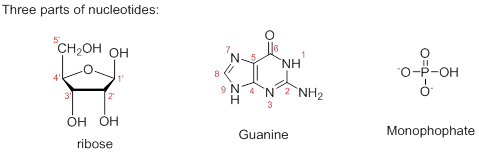
Therefore, the structure of given Guanosine 5’-monophosphate is,
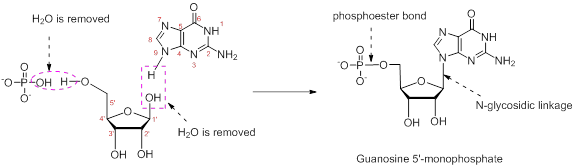
The ribonucleotide structure is predicted and named.
Want to see more full solutions like this?
Chapter 26 Solutions
EBK FUNDAMENTALS OF GENERAL, ORGANIC, A
Additional Science Textbook Solutions
Biological Science (6th Edition)
Biology: Life on Earth (11th Edition)
Genetics: From Genes to Genomes
Fundamentals of Anatomy & Physiology (11th Edition)
Chemistry & Chemical Reactivity
Campbell Essential Biology (7th Edition)
- Show the fate of the hydrogen on carbon-2 of glucose.arrow_forwardImagine that aldolase can react with the seven carbon molecule Sedoheptulose-1,7-bisphosphate (below). Use the mechanism to predict the two products generated.arrow_forwardShow the mechanisms of PGK and PFK-1. How are they different?arrow_forward
- Show the fate of the proton on the 4-Oxygen molecule of F-1,6-BP.arrow_forwardSodium borohydride (NaBH4) is a potent inhibitor of aldolase. It is known to ONLY inhibit theenzyme when it is complexed with substrate. Treatment of the enzyme alone has no effect.What is the mechanism for this inhibition?arrow_forwardA non-hydrolysable ATP (AMPPNP - below) is ingested by a graduate student on a dare. Whateffects would you anticipate on his metabolism?arrow_forward
- Show the mechanism for the acid-catalyzed formation of an [α-1,6] glycosidic linkagebetween two molecules of α-D-glucopyranose. Please sketch the structure and use arrows showing electron flow!arrow_forwardI am a Biochemistry student and I am confused on how to analyze FRAP Analysis using Excel Spread Sheets. The following spread sheet has my 0 minute data listed at top and the 4 minute data listed on the bottom. Sheet: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EXjrCizWiXRPmpittqZA12IB8EkB5eE8iaRqj_iun-IAtg?rtime=Wo9zPHFY3Ug The formula for FRAP Analysis is: FRAP value = A (4 min sample) - A (0 min sample) over A (4 min 30 uM ascorbic acid) - A (0 min 30 uM ascorbic acid) multiplied by 30 uM and the dilution factor of 1/10arrow_forwardHO Fill in the missing boxes. ON 800 NO NO Glucose ATP NADH Hexokinase 1,3-Bisphosphoglycerate Mg2+ ADP NAD+, Pi Phosphoglucose Isomerase Glucose-6-Phosphate ON 沁 Glyceraldehyde-3-Phosphate HO حلمة ADP ADP Phospho Mg2+ glycerate Dihydroxyacetone Phosphate ATP kinase ATP Phosphoglycerate 3-phosphoglycerate Mutase H₁₂O Fructose-6-Phosphate ATP Mg2+ ADP Fructose-1,6-Bisphosphate 2-phosphoglycerate H₂O Phosphoenolpyruvate ADP Mg2+ ATP Pyruvatearrow_forward
- In a diffraction experiment of a native crystal, intensity of reflection (-1 0 6) is equivalent to the intensity of reflection (1 0 -6). true or false?arrow_forwardin an x-ray diffraction experiment, moving the detector farther away from the crystal will allow collection of reflection of reflections with high Miller indices. true or false?arrow_forwardShow the mechanism for the acid-catalyzed formation of an [α-1,6] glycosidic linkagebetween two molecules of α-D-glucopyranose.arrow_forward
 Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning





