
(a)
Interpretation: Product of the given coupling reaction is to be predicted.
Concept introduction: Organocuprate reagents
Answer to Problem 26.1P
The product of the given coupling reaction is octane as shown below.
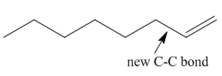
Explanation of Solution
Species containing copper-carbon bonds are known as organocuprate reagents. These reagents act as nucleophille and react with organic halides to form coupling products that contains new
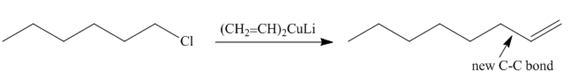
Figure 1
Product formed from the coupling reaction is shown in Figure 1.
(b)
Interpretation: Product of the coupling reaction is to be predicted.
Concept introduction: Organocuprate reagents
Only one alkyl group of the organocuprate reagent is transferred to form the product, while the other becomes part of reaction’s by-product.
Answer to Problem 26.1P
The product of the coupling reaction is
Explanation of Solution
Species containing copper-carbon bonds are known as organocuprate reagents. These reagents act as nucleophille and react with organic halides to form coupling products that contains new
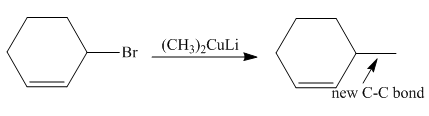
Figure 2
Product formed from the coupling reaction is shown in Figure 2.
(c)
Interpretation: Product of the coupling reaction is to be predicted.
Concept introduction: Organocuprate reagents
Only one alkyl group of the organocuprate reagent is transferred to form the product, while the other becomes part of reaction’s by-product.
Answer to Problem 26.1P
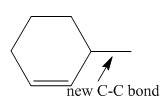
The product of the coupling reaction is
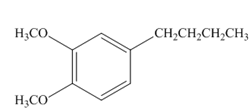
Explanation of Solution
Species containing copper-carbon bonds are known as organocuprate reagents. These reagents acting as nucleophille and react with organic halides to form coupling products that contains new
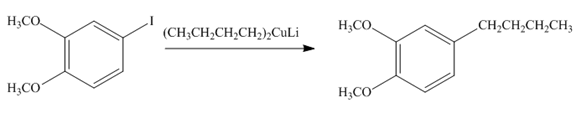
Figure 3
Product formed from the coupling reaction is shown in Figure 3.
(d)
Interpretation: Product of the coupling reaction is to be predicted.
Concept introduction: Organocuprate reagents
Only one alkyl group of the organocuprate reagent is transferred to form the product, while the other becomes part of reaction’s by-product.
Answer to Problem 26.1P
The product of the coupling reaction is
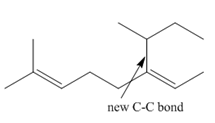
Explanation of Solution
Species containing copper-carbon bonds are known as organocuprate reagents. These reagents act as nucleophille and react with organic halides to form coupling products that contains new
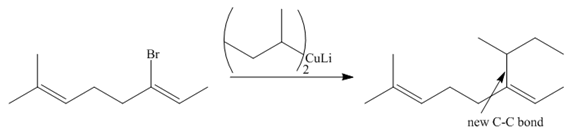
Figure 4
Product formed from the coupling reaction is shown in Figure 4.
Want to see more full solutions like this?
Chapter 26 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
- Draw a Lewis structure for each of the following species. Again, assign charges where appropriate. a. H-H¯ b. CH3-CH3 c. CH3+CH3 d. CH3 CH3 e. CH3NH3+CH3NH3 f. CH30-CH3O¯ g. CH2CH2 - h. HC2-(HCC) HC2 (HCC) i. H202×(HOOH) H₂O₂ (HOOH) Nortonarrow_forwardIs molecule 6 an enantiomer?arrow_forwardShow work. Don't give Ai generated solutionarrow_forward
- Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 Molecule 3 ----||| Molecule 4 Molecule 5 Molecule 6 none of the above mm..arrow_forwardShow work. don't give Ai generated solutionarrow_forwardCheck the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 Molecule 3 ----||| Molecule 4 Molecule 5 Molecule 6 none of the above mm..arrow_forward
- Use the vapor-liquid equilibrium data at 1.0 atm. for methanol-water (Table 2-8 ) for the following: If the methanol vapor mole fraction is 0.600, what is the methanol liquid mole fraction? Is there an azeotrope in the methanol-water system at a pressure of 1.0 atmospheres? If water liquid mole fraction is 0.350, what is the water vapor mole fraction? What are the K values of methanol and of water at a methanol mole fraction in the liquid of 0.200? What is the relative volatility αM-W at a methanol mole fraction in the liquid of 0.200?arrow_forwardCheck the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. || |II***** Molecule 1 | Molecule 4 none of the above Molecule 2 Molecule 3 Х mm... C ---||| *** Molecule 5 Molecule 6arrow_forwardis SiBr4 Silicon (IV) tetra Bromine? is KClO2 potassium dihypochlorite ?arrow_forward
- "יוון HO" Br CI Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 Molecule 3 Br Br Br HO OH H CI OH ✓ Molecule 4 Molecule 5 Molecule 6 CI Br יייון H Br OH OH CI Br ☐ none of the above × Garrow_forwardUS2 Would this be Uranium (II) diSulfide?arrow_forwardnomenclature for PU(SO4)3arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





