
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
5th Edition
ISBN: 9781260170405
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 26, Problem 26.51P
Devise a synthesis of each of the following compounds. Besides inorganic reagents, you may use hydrocarbons and halides having
a. 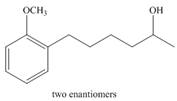 c.
c. 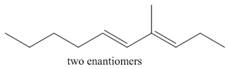
b. 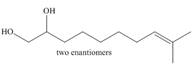 d.
d. 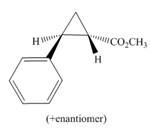
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Identify the Functional Groups (FG) in the following molecules. Classify C atoms as tertiary, 30, or quaternary 40. Identify secondary 20 and tertiary, 30 hydrogen atoms. Please provide steps to undertand each labeling. Please label in the image, so it fits explanation. I am still very unsure I undertand this.
Don't used Ai solution
Don't used Ai solution
Chapter 26 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
Ch. 26 - Prob. 26.1PCh. 26 - Prob. 26.2PCh. 26 - Prob. 26.3PCh. 26 - Prob. 26.4PCh. 26 - Prob. 26.5PCh. 26 - Prob. 26.6PCh. 26 - Prob. 26.7PCh. 26 - Problem 26.8
What starting materials are needed to...Ch. 26 - Prob. 26.9PCh. 26 - Problem 26.10
What reagents are needed to convert...
Ch. 26 - Problem 26.11
What product is formed when each...Ch. 26 - Prob. 26.12PCh. 26 - Problem 26.13
Draw the products formed when each...Ch. 26 - Problem 26.14
What products are formed when ...Ch. 26 - Problem 26.15
Draw the product formed from...Ch. 26 -
What product is formed by ring-closing metathesis...Ch. 26 - Problem 26.17
What starting material is needed to...Ch. 26 - 26.18 In addition to organic halides, alkyl...Ch. 26 - 26.19 What product is formed by ring-closing...Ch. 26 - 26.20 Draw the products formed in each...Ch. 26 - What organic halide is needed to convert lithium...Ch. 26 - 26.22 How can you convert ethynylcyclohexane to...Ch. 26 - 26.23 What compound is needed to convert styrene...Ch. 26 - 26.24 What steps are needed to convert to octane...Ch. 26 - Prob. 26.25PCh. 26 - Prob. 26.26PCh. 26 - 26.27 Draw the products (including stereoisomers)...Ch. 26 - 26.28 Treatment of cyclohexene with and forms...Ch. 26 - Prob. 26.29PCh. 26 - 26.30 What starting material is needed to prepare...Ch. 26 - Prob. 26.31PCh. 26 - Prob. 26.32PCh. 26 - When certain cycloalkenes are used in metathesis...Ch. 26 - 26.34 Draw the products formed in each reaction.
...Ch. 26 - Prob. 26.35PCh. 26 - Draw a stepwise mechanism for the following...Ch. 26 - Sulfur ylides, like the phosphorus ylides of...Ch. 26 - Although diazomethane is often not a useful...Ch. 26 - Prob. 26.39PCh. 26 - Prob. 26.40PCh. 26 - Prob. 26.41PCh. 26 - Prob. 26.42PCh. 26 - 26.43 Devise a synthesis of each compound using a...Ch. 26 - 26.44 Devise a synthesis of each compound from...Ch. 26 - 26.45 Devise a synthesis of each compound from...Ch. 26 - 26.46 Devise a synthesis of each substituted...Ch. 26 - Biaryls, compounds containing two aromatic rings...Ch. 26 - Prob. 26.48PCh. 26 - 26.49 Draw the product formed from the...Ch. 26 - Prob. 26.50PCh. 26 - 26.51 Devise a synthesis of each of the following...Ch. 26 - Prob. 26.52PCh. 26 - 26.53 The following conversion, carried out in the...Ch. 26 - Prob. 26.54PCh. 26 - 26.55 Dimethyl cyclopropanes can be prepared by...Ch. 26 - Prob. 26.56P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3. Devise a retrosynthesis for the problem given below and then provide the corresponding synthesis with all necessary reagents/reactants: RETROSYNTHESIS: SYNTHESIS: Brarrow_forwardSeveral square planar complexes are known for Gold (III) ions but not for Silver (III) why?arrow_forwardAiter running various experiments, you determine that the mechanism for the following reaction is bimolecular. CI Using this information, draw the correct mechanism in the space below. X Explanation Check C Cl OH + CI Add/Remove step Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Carrow_forward
- Complete the reaction in the fewest number of steps as possible, Draw all intermediates (In the same form as the picture provided) and provide all reagents.arrow_forwardPlease provide steps to work for complete understanding.arrow_forwardPlease provide steps to work for complete understanding.arrow_forward
- Identify the Functional Groups (FG) in the following molecules. Classify C atoms as tertiary, 30, or quaternary 40. Identify secondary 20 and tertiary, 30 hydrogen atoms. Please provide steps to undertand each labeling.arrow_forwardIdentify the Functional Groups (FG) in the following molecules. Classify C atoms as tertiary, 30, or quaternary 40. Identify secondary 20 and tertiary, 30 hydrogen atoms. Please provide steps to undertand each labeling.arrow_forwardIdentify the Functional Groups (FG) in the following molecules. Classify C atoms as tertiary, 30, or quaternary 40. Identify secondary 20 and tertiary, 30 hydrogen atoms. Please provide steps to undertand each labeling.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY